Reference Laboratories
The Reference Laboratories are designated to pursue all the scientific and technical problems relating to a named disease. The Expert, responsible to WOAH and its Member Countries with regard to the disease, should be a leading and active researcher helping the Reference Laboratory to provide scientific and technical assistance and expert advice on topics linked to diagnosis and control of the disease for which the Reference Laboratory is responsible. Reference Laboratories should also provide scientific and technical training for personnel from Member Countries, and coordinate scientific and technical studies in collaboration with other laboratories or organisations, including through the Laboratory Twinning programme.
- To use, promote and disseminate diagnostic methods validated according to WOAH Standards;
- To recommend the prescribed and alternative tests or vaccines as WOAH Standards;
- To develop reference material in accordance with WOAH requirements, and implement and promote the application of WOAH Standards;
- To store and distribute to national laboratories biological reference products and any other reagents used in the diagnosis and control of the designated pathogens or diseases;
- To develop, standardise and validate according to WOAH Standards new procedures for diagnosis and control of the designated pathogens or diseases;
- To provide diagnostic testing facilities, and, where appropriate, scientific and technical advice on disease control measures to WOAH Member Countries;
- To carry out and/or coordinate scientific and technical studies in collaboration with other laboratories, centres or organisations;
- To collect, process, analyse, publish and disseminate epizootiological data relevant to the designated pathogens or diseases;
- To provide scientific and technical training for personnel from WOAH Member Countries;
- To maintain a system of quality assurance, biosafety and biosecurity relevant for the pathogen and the disease concerned;
- To organise and participate in scientific meetings on behalf of the WOAH;
- To establish and maintain a network with other WOAH Reference Laboratories designated for the same pathogen or disease and organise regular inter-laboratory proficiency testing to ensure comparability of results;
- To organise inter-laboratory proficiency testing with laboratories other than WOAH Reference Laboratories for the same pathogens and diseases to ensure equivalence of results;
- To place expert consultants at the disposal of the WOAH.
1. Scope and background
In May 2011, the World Assembly of Delegates of the WOAH (hereafter the Assembly) adopted new Terms of Reference (ToRs) and Internal Rules for WOAH Reference Centres. The ToRs for Reference Laboratories had emphasised their role in developing and recommending test methods, storing and distributing reference reagents, providing advice, diagnostic support and training to WOAH Members, and their reporting obligations. From 2011, the ToRs added the recommendation that laboratories establish and maintain a network with other WOAH Reference Laboratories designated for the same pathogen or disease and organise regular inter-laboratory proficiency testing to ensure comparability of results, as well as organise inter-laboratory proficiency testing with laboratories other than WOAH Reference Laboratories for the same pathogens and diseases to ensure equivalence of results.
WOAH Reference Laboratories are designated to pursue scientific and technical problems relating to a named disease or pathogen. The designated Expert should be a leading member of a multidisciplinary team helping the Reference Laboratory to provide scientific and technical assistance and expert advice on diagnosis and control of the disease or pathogen for which the Reference Laboratory is responsible. Reference Laboratories should also provide scientific and technical training for personnel from Member Countries, and coordinate scientific and technical studies in collaboration with other laboratories or organisations, including through WOAH Laboratory Twinning.
The integrity and credibility of the WOAH is intimately linked to the quality of the science to which it has access. The WOAH depends very heavily on its designated Reference Laboratories and disease experts for scientific advice and support, both to the WOAH Headquarters in developing standards, participating in ad hoc Groups and providing general advice, and to individual Members.
The WOAH has developed this document on the Procedures for designation of WOAH Reference Laboratories to assist Members, current WOAH Reference Laboratories and experts, and applicant laboratories to better understand the applicable procedures.
2. Submission of an application
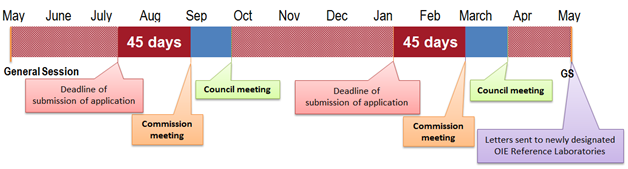
The WOAH work programme cycle runs from May to May, of which the General Sessions of the Assembly are the start and end points. There are two Specialist Commissions responsible for evaluating WOAH Reference Laboratory applications: Biological Standards Commission and Aquatic Animal Health Standards Commission for WOAH Reference Laboratories for terrestrial and aquatic animal diseases, respectively. These Commissions meet twice in a cycle, with the first meeting usually held August/September and the second meeting in February/March; these dates can vary each cycle based on the availability of the members of the relevant Commissions (cf. Figure 1).
Applications should be submitted 45 days before the date scheduled for the meetings of the relevant Commission. The 45-day period gives the WOAH sufficient time to screen, translate into English when necessary, and process the dossiers for the Commission’s evaluation. Deadlines must be strictly observed to allow a full evaluation of the dossiers by the members of the Commission prior to the meeting. Applications received after the deadline are examined at the next Commission meeting.
The applicant laboratory should submit the information using the guidelines for applicants for WOAH Reference Laboratory status published on the WOAH website: https://www.woah.org/en/what-we-offer/expertise-network/reference-laboratories/#ui-id-8. Applications must be limited to no more than 20 pages in A4 format, single-spaced using Times New Roman font size 10pt. Relevant appendices may be attached with clear cross-referencing to the core document. All documents must be prepared in one of the official languages of the WOAH (English, French or Spanish).
While evaluating a submitted dossier, the Commission may have questions for the applicant laboratory. These questions will be sent by letter signed by the Director General of the WOAH after the Commission meeting. The applicant laboratory should provide written answers by an appointed deadline or by the deadline prior to the next meeting of the Commission (45 days before the date scheduled for the next meeting of the relevant Commission).
3. Preliminary screening of application
On submission of the dossier, the WOAH Headquarters (Science Department) acknowledges its receipt and confirms the meeting dates of the relevant Commission. If a gap in the information provided is identified, the WOAH Headquarters may request the submission of an amended application or additional information before a set deadline.
4. Evaluation by the relevant WOAH Specialist Commissions
As stated previously, the Biological Standards Commission and the Aquatic Animal Health Standards Commission conduct evaluations of WOAH Reference Laboratory applications for terrestrial and aquatic animal diseases, respectively.
The Terms of Reference, Internal Rules, Qualification and election procedures of members of the Commissions are found in the WOAH Basic Texts. The members of the Commissions are elected or re-elected every 3 years by the Assembly.
Commission members are requested to comply with the WOAH requirements and procedures regarding confidentiality and the management of conflicts of interest. The President of the Commission and the WOAH Secretariat ensure that any members with conflicting interests in relation to a particular dossier do not take part in the discussions and final decision-making.
In accordance with the criteria for designation as an WOAH Reference Centre listed in the WOAH Basic Texts, and Resolutions adopted at each General Session with regard to the designation of WOAH Reference Laboratories for terrestrial and aquatic animal diseases, all applications are assessed using standardised principles that include: the institution’s ability, capacity and readiness to provide services; the scientific and technical standing of the institution concerned at the national and international levels; the quality of its scientific and technical leadership including internationally recognised expertise; the institution’s prospective stability in terms of personnel, activity and funding; and the technical and geographical relevance of the institution and its activities to the WOAH’s programme priorities.
When conducting an evaluation of an applicant WOAH Reference Laboratory, the Commission may also take into account any other information available in the public domain that is considered as pertinent to the evaluation of the dossier.
In accordance with the Basic Texts of the WOAH, all formal correspondence between the Commission and outside individuals or bodies shall be issued through the office of the Director General of the WOAH. All correspondence between an applicant laboratory and the WOAH Headquarters is duly documented by the WOAH Headquarters.
5. Endorsement by the WOAH Council
In accordance with Article 3 of Chapter 4 on the Internal Rules and relevant Resolutions previously adopted, all WOAH Reference Laboratory applications are endorsed by the WOAH Council before presented to the Assembly for approval.
6. Communication on the outcome of the evaluation with the applicant laboratory
After its meeting, the Commission produces a report that includes the outcomes of the evaluation of Reference Laboratory applications. The identity of the applicant laboratory is published in the report along with the recommendation that it be accepted by the Assembly for adoption by resolution. Unsuccessful applicants are informed by letter from the Director General of the WOAH. This letter is not released in the public domain and the identity of the laboratory is not revealed in the Commission report. In some cases, the Commission may have questions or require additional information before a final decision can be taken. This information should be submitted to the WOAH by the appointed deadline for consideration by the Commission at its next meeting.
7. Designation of WOAH Reference Laboratories by the Assembly
The Assembly, on the basis of the assessment by the relevant WOAH Commission and the endorsement by the WOAH Council, adopts by Resolution all new WOAH Reference Laboratories. Official designation as an WOAH Reference Laboratories comes into force only after adoption by Resolution of the Assembly.
Shortly after the General Session, the newly designated WOAH Reference Laboratory will receive a letter from the Director General of the WOAH. The WOAH Headquarters also updates the list of Reference Experts and Laboratories on its website.
8. Change of the WOAH Reference Laboratory expert
In accordance with Resolution No. 34 adopted at the 81st General Session in May 2013, the Assembly delegated to the Council the authority to approve, on its behalf, the replacement of WOAH designated Experts at existing WOAH Reference Laboratories, provided that the nominations submitted by the head of the Reference Laboratory through the WOAH Delegate of the country of location have been examined and endorsed by the relevant WOAH Specialist Commission.
If the expert decides to relinquish the title of WOAH designated expert and if the laboratory wishes to maintain its WOAH Reference Laboratory status, an official letter – detailing the situation and enclosing a nomination for a replacement expert, including a curriculum vitae together with documentation of his or her work related on the disease or pathogen – should be submitted to the WOAH through the Delegate of the country. The nomination will be considered by the relevant WOAH Specialist Commission at its next meeting, and the decision will be notified to the WOAH Reference Laboratory. The official change of WOAH Reference Laboratory expert will take place only after the approval of the Council.
Given the meeting schedules of the Specialist Commissions and the Council, the possibility exists that an WOAH Reference Laboratory could temporarily have no designated expert. The WOAH expects that, under normal circumstances, Reference Laboratories will always have an WOAH designated expert in place and will plan ahead to take into account retirement or resignation. Should the Specialist Commission not endorse a nomination for a replacement expert, the Reference Laboratory will have until the following Commission meeting to submit or re-submit a nomination. During the time between meetings, the Reference Laboratory will remain on the WOAH list with the words “To be decided” replacing the name of the expert. The laboratory will need to provide a working email address to accompany the entry on the WOAH list. If at the second meeting the Reference Laboratory either does not submit a new or renewed nomination, or the nomination is not endorsed by the Commission, the Reference Laboratory will be suspended and removed from the WOAH list. The Reference Laboratory will then have 1 year (two consecutive Specialist Commission meetings) to successfully fill the position of replacement expert and be reinstated on the WOAH List. If after 1 year from the initial removal from the list, no nominee has been endorsed and the position is thus vacant, the Reference Laboratory designation will the withdrawn in accordance with Article 9 of the Internal Rules (cf. Section 10).
9. Suspension of Reference Laboratory status
WOAH Reference Laboratories are expected to fulfil their ToRs and Internal Rules. They must have an approved designated expert responsible for the implementation of the technical aspects of the ToRs. Should a Reference Laboratory find that it is unable to fulfil the ToRs for a temporary period, for example due to the absence of a succession strategy resulting in the lack of an approved designated expert or temporary lack of diagnostic ability due to construction or restructuring of the laboratory’s facilities, the Reference Laboratory should inform the WOAH Headquarters immediately of the situation. The WOAH Headquarters, in consultation with the relevant Specialist Commission, may decide to temporarily suspend the laboratory’s WOAH status until the laboratory can operate to the standard required of WOAH Reference Laboratories. The period of suspension should be no longer than 2 years. During that period, the laboratory will be removed from the WOAH list. At any time during the 2-year period, the laboratory’s status could be reinstated upon receipt and acceptance by the relevant Specialist Commission of proof that the Reference Laboratory is operational to the required standard again. If in the 2-year period, the laboratory cannot provide proof of its operational ability, it designation shall be withdrawn in accordance with Article 9 of the Internal Rules (cf. Section 10).
10. De-listing of WOAH Reference Laboratories
Upon the screening and analysis performed by the WOAH Headquarters (cf. Section 11.1.), the relevant Commission reviews the reports and activities of the Reference Laboratories. Where there is insufficient evidence of WOAH mandate-related activities, the Commission may recommend to the Council and to the Assembly the withdrawal of the Reference Laboratory designation.
In accordance with Article 9 of the Internal Rules, a Reference Laboratory may revoke the designation at any time. If an WOAH Reference Laboratory decides to withdraw its designation as such, an official letter should be submitted to the WOAH through the Delegate of the country.
Moreover, in accordance with Article 9 of the Internal Rules, the designation of a Reference Laboratory shall be withdrawn if the Reference Laboratory fails to comply with the provisions of the ToRs and the present Rules. In such cases, the Director General of the WOAH, after consulting the appropriate WOAH Specialist Commission and WOAH Council and notifying the Delegate of the country, proposes the withdrawal to the Assembly.
In 2016, the Specialist Commissions and the Director General of the WOAH, identified five critical points for consideration when evaluating a laboratory’s performance:
i) the lack of submission of annual report;
ii) the lack of accreditation to ISO 17025 or equivalent quality management system, ideally with relevant tests included in the scope of the accreditation;
iii) a pattern revealing lack of diagnostic activity or production and supply of reference material related to the disease or pathogen;
iv) no response to requests from the WOAH Headquarters for scientific expertise (e.g. inquiry of technical advice from WOAH Member Countries, revision of the Terrestrial manual chapters, etc.).
v) no response to requests from the WOAH for administrative issues relating to transparency and confidentiality (e.g. not renewing the potential conflict of interests declaration or providing a confidentiality undertaking: https://www.woah.org/en/who-we-are/structure/framework/).
11. WOAH Reference Laboratory Annual report
In accordance with Article 8 of the Internal Rules, the Reference Centre shall provide to the Director General a brief report of activities related to their ToRs at the end of each calendar year, according to the template established by the WOAH Headquarters. A letter from the Director General of the WOAH is sent to all designated experts of WOAH Reference Laboratories for submission of the annual report.
Since December 2013, an on-line system for submitting annual reports the WOAH Reference Laboratories has been in place.
The template of the annual report is structured around each ToR for WOAH Reference Laboratories as adopted in May 2011. Questions are close-ended (yes/no answers) to generate more accurate and comparable information from the laboratories. Tables to allow for the collection of detailed information related to the activities carried out by the laboratories are also included. The on-line annual reporting system can be accessed via a dedicated link and a randomly generated username and password that are sent to all Experts of WOAH Reference Laboratories in a letter signed by the Director General of the WOAH during the last month of the reporting year. The deadline to submit the annual report of the WOAH Reference Laboratory activities of each calendar year is usually by mid-January of the following year.
11.1. Review and analysis of the annual reports
The submitted annual reports are first screened and quantitatively analysed, based on the close-ended (yes/no) answers, by the WOAH Headquarters. An overview of the analysis is presented to the relevant Commission at its February/March meeting.
WOAH Reference Laboratories are expected to fulfil the ToRs adopted by the WOAH World Assembly of Delegates as reflected in the annual report.
Any questions or concerns that may arise during the review of annual reports by the Commission can be referred to the concerned WOAH Reference Laboratory through the office of the Director General of the WOAH.
All annual reports of WOAH Reference Laboratories are made available to all Member Countries on the WOAH website (https://www.woah.org/en/what-we-offer/expertise-network/reference-laboratories/#ui-id-5) shortly after the February meeting of the Commissions.
11.2. Lack of submission of the annual report
After the meeting of the relevant Commissions, laboratories that have not submitted their annual reports will be sent a letter of reminder, with the Delegate of the host Member Country in copy, to submit the report by an extended and prescribed deadline. For the laboratories that have still not submitted an annual report by the end of March, a reminder will be addressed directly to the Delegate, with the expert in copy, giving a 2-week deadline to reply to the WOAH with an explanation of the situation or circumstances that may have prevented the laboratory from fulfilling this ToR.
Further communication by letter or direct communication during the General Session may be considered, if needed, prior to the final recommendation to de-list the laboratory, which would be taken by the Commission at the September meeting. This procedure could also be applied to laboratories falling under one of the four other de-listing criteria (cf. Section 10).
Contact: [email protected]
Acute hepatopancreatic necrosis disease
-
Prof. Han-Ching Wang
CHINESE TAIPEI
Address
International Center for the Scientific Development of Shrimp Aquaculture (ICDSA), National Cheng Kung University (NCKU)
No. 500, Sec. 3, Anming Road, Annan District, Tainan City 709Contact details
+886-6 384 24 48
[email protected]
-
Dr Arun Dhar
UNITED STATES OF AMERICA
Address
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E Lowell St, Building 90, 85721 TucsonContact details
+1-520 621 87.27
[email protected]
African horse sickness
-
Dr Baratang Alison Lubisi
SOUTH AFRICA
Address
Senior Research Veterinarian
Onderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 91 17
[email protected]
-
Dr José Manuel Sánchez-Vizcaíno
SPAIN
Address
Centro de Vigilancia Sanitaria Veterinaria (VISAVET) Facultad de VeterinariaHCV Planta sótanoUniversidad Complutense de Madrid (UCM)
Avda Puerta de Hierro s/n, 28040 MadridContact details
+34-91 394.40.82
[email protected]
-
Dr Montserrat Agüero Garcia
SPAIN
Address
Laboratorio Central de Sanidad Animal
LCV-Algete, Ctra. Algete Km 828110 Algete, MadridContact details
+34 913 47 83 12
[email protected]
-
Dr. Carrie Batten
UNITED KINGDOM
Address
Pirbright
Ash Road, Woking, Surrey GU24 0NFContact details
+44-1483 23 24 41
[email protected]
African swine fever
-
Dr David Williams
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Geelong, Victoria 3220Contact details
+61 52.27.50.00
[email protected]
-
Dr Aruna Ambagala
CANADA
Address
National Centre for Foreign Animal Disease, Canadian Food Inspection Agency
1015 Arlington Street, Winnipeg, Manitoba R3E 3M4Contact details
+12047892089
[email protected]
[email protected]
-
Dr Zhiliang Wang
PEOPLE'S REPUBLIC OF CHINA
Address
National Surveillance and Research Center for Exotic Animal Diseases
China Animal Health and Epidemiology Center, 369 Nanjing Road, Qingdao 266032Contact details
+86-532 85.63.91.66
[email protected]
[email protected]
-
Dr Livio Heath
SOUTH AFRICA
Address
Onderstepoort Veterinary Institute,
Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 95.01
[email protected]
-
Dr José Manuel Sánchez-Vizcaíno
SPAIN
Address
Centro de Vigilancia Sanitaria Veterinaria (VISAVET) Facultad de VeterinariaHCV Planta sótanoUniversidad Complutense de Madrid (UCM)
Avda Puerta de Hierro s/n, 28040 MadridContact details
+34-91 394.40.82
[email protected]
-
Dr Linda Dixon
UNITED KINGDOM
Address
Ash Road, Pirbright Woking, Surrey, GU24 0NF Pirbright
Contact details
+44-1483 23 24 41
[email protected]
-
Dr. Ping Wu
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
40550 Route 25, Orient, New York, NY 11957Contact details
+16313233287
[email protected]
American foulbrood (infection of honey bees with Paenibacillus larvae)
-
Dr Marie-Pierre Chauzat
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia Antipolis Laboratory, Honey Bee Pathology Unit, Les Templiers 105 route des Chappes, CS 20111, 06902 Sophia AntipolisContact details
+33 (0)4 92 94 37 00
[email protected]
-
Dr Marc O. Schäfer
GERMANY
Address
National Reference Laboratory for Bee Diseases, Friedrich-Loeffer-Institut
Federal Research Institute for Animal Health, Institute of Infectology, Südufer 1017493 Greifswald – Insel RiemsContact details
+49-38351 7 1246
[email protected]
-
Dr. Richard J. Hall
NEW ZEALAND
Contact details
+6448945600
[email protected]
Anaplasmosis
-
Dr Juan Joel Mosqueda Gualito
MEXICO
Address
Centro Nacional de Servicios de Constatación en Salud Animal (CENAPA)
Carretera Cuernavaca Cuautla #8534 Colonia Progreso CB 62550, Jiutepec Morelos MorelosContact details
+52-777 3.19.02.02
[email protected]
Anthrax
-
Dr. Kingsley Amoako
CANADA
-
Ms Ginger Harvey
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratory, USDA, APHIS, VS
1920 Dayton Avenue, Ames, Iowa 50010Contact details
+15153377070
[email protected]
Antimicrobial resistance
-
Dr Chris Teale
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1743 46 76 21
[email protected]
Aujeszky's disease
-
Dr Marie-Frédérique Le Potier
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Ploufragan-Plouzané-Niort Laboratory, Porcine Virology Immunology Unit, B.P. 53, 22440 PloufraganContact details
+33 (0)2 96 01 62 90
[email protected]
Avian chlamydiosis
-
Dr Karine Laroucau
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, 94701 Maisons-Alfort CedexContact details
+330149771350
[email protected]
-
Dr Christiane Schnee
GERMANY
Address
Institute of Molecular PathogenesisFriedrich-Loeffler-InstituteFederal Research Institute for Animal Health
Naumburger Str. 96a 07743 Jena JenaContact details
+49-3641 804 2435
[email protected]
Avian influenza
-
Dr. Frank Wong
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 50 00
[email protected]
[email protected]
-
Dr Dilmara Reischak
BRAZIL
Address
Laboratório Federal de Defesa Agropecuária em Sao Paulo – LFDA-SPUnidade de Sanidade Aviária
Rua Raul Ferrari, s/n° Jardim Santa Marcelina CEP 13100-105 Campinas SP Sao PauloContact details
+55-19 32.52.31.74
[email protected]
-
Dr. Yohannes Berhane
CANADA
Address
Canadian Food Inspection AgencyNational Centre for Foreign Animal Disease
1015 Arlington Street Winnipeg, Manitoba R3E 3M4 WINNIPEGContact details
+1-204 789 20 03
[email protected]
-
Dr Abdelsatar Arafa
EGYPT
Address
Reference Laboratory for Veterinary Quality Control on Poultry Production
Animal Health Research Institute, Agriculture research Centre, Ministry of Agriculture and Land Reclamation, 7 Nadi el Seidst. Dokki, GizaContact details
+202-33 37.09.58
[email protected]
-
Dr Timm C. Harder
GERMANY
Address
Friedrich Loeffler InstituteFederal Research Institute for Animal HealthInstitute of Diagnostic Virology
Südufer 10 D-17493 Greifswald Insel Riems GREIFSWALDContact details
+49-38351 7 1152
[email protected]
-
Dr Chakradhar Tosh
INDIA
Address
Indian Council of Agricultural Research (ICAR), National Institute of High Security Animal Diseases (NIHSAD)
Anand Nagar, Bhopal 462 021 Madhya Pradesh, BhopalContact details
+91-755 275 92 04
[email protected]
[email protected]
-
Dr Isabella Monne
ITALY
Address
Istituto Zooprofilattico Sperimentale delle Venezie, Research and Innovation Dept.
Viale Dell'Università 10, 35020 Legnaro PDContact details
+39-049 808 4381
[email protected]
-
Prof. Yoshihiro Sakoda
JAPAN
Address
Hokkaido University, Research Center for Zoonosis Control
North 20, West 10 Kita-Ku, Sapporo 001-0020Contact details
+81-11 706 52 07
[email protected]
-
Dr. Eun-Kyoung Lee
KOREA (REP. OF)
Address
Animal and Plant Quarantine Agency
Ministry of Agriculture, Forest and Rural Affairs, 177, Hyeoksin 8-ro, Gimcheon-si, Gyeongsangbuk-do 39660Contact details
+82549120968
[email protected]
-
Dr Hualan Chen
PEOPLE'S REPUBLIC OF CHINA
Address
National Avian Influenza Reference Laboratory, Animal Influenza Laboratory of the Ministry of Agriculture
Harbin Veterinary Research Institute, 427 Maduan Street, Harbin 150001Contact details
+86-451 85.93.50.79
[email protected]
-
Dr. Viktor N. Irza
RUSSIA
Address
National Reference Laboratory for Avian Influenza and Newcastle Disease
Federal State-Financed Institution “Federal Centre for Animal Health” (FGBI “ARRIAH”), Yur'evets, Vladimir 60090Contact details
+7-4922 26 18 67
[email protected]
[email protected]
-
To Be Decided
UNITED KINGDOM
Address
WOAH/FAO International Reference Laboratory for Avian Influenza
Animal and Plant Health Agency – Weybridge, Addlestone, Surrey KT15 3NBContact details
+441483232441
[email protected]
[email protected]
-
Dr. Mia Kim Torchetti
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
1920 Dayton Avenue, Ames, Iowa 50010Contact details
+15153377551
[email protected]
Avian mycoplasmosis (Mycoplasma gallisepticum)
-
To be decided
CUBA
Address
MYCOLAB Laboratorio para diagnóstico de micoplasmas, Centro Nacional de Sanidad Agropecuaria
San José de las Lajas Provincia Mayabeque CUBAContact details
+53-47 86.33.14 ext. 153
[email protected]
-
Dr. Salvatore Catania
ITALY
-
Dr. Umit Özdemir
REPUBLIC OF TÜRKIYE
Address
Pandik Veterinary Control Institute
Batı mahallesi, Erol Kaya caddesi 1, 34890 İstanbulContact details
+90-216 390.12.80
[email protected]
Avian mycoplasmosis (Mycoplasma synoviae)
-
To be decided
CUBA
Address
MYCOLAB Laboratorio para diagnóstico de micoplasmas, Centro Nacional de Sanidad Agropecuaria
San José de las Lajas Provincia Mayabeque CUBAContact details
+53-47 86.33.14 ext. 153
[email protected]
-
Dr. Salvatore Catania
ITALY
-
Dr. Umit Özdemir
REPUBLIC OF TÜRKIYE
Address
Pandik Veterinary Control Institute
Batı mahallesi, Erol Kaya caddesi 1, 34890 İstanbulContact details
+90-216 390.12.80
[email protected]
Babesiosis
-
Dr Valeria Blanda
ITALY
Address
Italian Reference Centre for Anaplasma, Babesia, Rickettsia, Theileria (C.R.A.Ba.R.T.)
Istituto Zooprofilattico Sperimentale della Sicilia (IZSSi), via Gino Marinuzzi 3, 90129, PalermoContact details
+39-091 656.53.41 ext 219
[email protected]
-
Dr Juan Joel Mosqueda Gualito
MEXICO
Address
Centro Nacional de Servicios de Constatación en Salud Animal (CENAPA)
Carretera Cuernavaca Cuautla #8534 Colonia Progreso CB 62550, Jiutepec Morelos MorelosContact details
+52-777 3.19.02.02
[email protected]
Bluetongue
-
Dr Debbie Eagles
AUSTRALIA
Address
CSIRO
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 00 00
[email protected]
-
Dr Giovanni Savini
ITALY
Address
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale"
Via Campo Boario, 64100 TeramoContact details
+39-0861 33 24 40
[email protected]
-
Dr Baratang Alison Lubisi
SOUTH AFRICA
Address
Senior Research Veterinarian
Onderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 91 17
[email protected]
-
Dr. Carrie Batten
UNITED KINGDOM
Address
Pirbright
Ash Road, Woking, Surrey GU24 0NFContact details
+44-1483 23 24 41
[email protected]
Bovine babesiosis
-
Prof. Naoaki Yokoyama
JAPAN
Address
Obihiro University of Agriculture and Veterinary Medicine Nishi 2-13, Inada-cho Obihiro, Hokkaido 080-8555 Hokkaido
Contact details
+81-155 49.56.49
[email protected]
Bovine spongiform encephalopathy
-
Dr. Waqas Tahir
CANADA
Address
Lethbridge Laboratory
Canadian Food Inspection Agency, National Centre for Animal Disease (NCAD), Lethbridge Laboratory, Lethbridge T1J 3Z4Contact details
+1-403 382.55.49
[email protected]
-
Dr. Cristina Casalone
ITALY
Address
Italian National Reference Centre for Diagnostic Activities in Stranded Marine Mammals (C.Re.Di.Ma.), via Bologna 148, 10154 TorinoContact details
+39-11 26 86 296
[email protected]
[email protected]
-
Dr. Yoshifumi Iwamaru
JAPAN
Address
National Agricultural Research Organization, Prion Diseases Research Unit, National Institute of Animal Health
3-1-5 Kannondai, Tsukuba, Ibaraki 305-0856Contact details
+81-29 838 83 33
[email protected]
-
Dr Juan José Badiola Díez
SPAIN
Address
Centro de investigación en Encefalopatías y enfermedades transmisibles emergentes
Universidad de Zaragoza, Facultad de Veterinaria, Departamento de Patología Animal, Miguel Servet 177, 50013 ZaragozaContact details
+34-976 76 20 19
[email protected]
-
Prof. Torsten Seuberlich
SWITZERLAND
Address
Neuro Centre Department of Clinical Research and Veterinary Public Health
Division of Experimental Clinical Research, University of Bern, Bremgartenstrasse 109 A3012 Bern,Contact details
+41-31 631 22 06
[email protected]
-
Dr John Spiropoulos
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44 1932 357.564
[email protected]
Bovine viral diarrhoea
-
Dr. Peter Kirkland
AUSTRALIA
Address
Elizabeth Macarthur Agriculture Institute (EMAI), Virology Laboratory
Woodbridge Rd, Menangle, PMB 8, Camden NSW 2570Contact details
+61-2 46.40.63.31
[email protected]
[email protected]
-
Dr. Oliver Lung
CANADA
Address
Canadian Food Inspection Agency,
National Centre for Animal Disease (NCAD), P.O. Box 640, Township Road 9-1, Lethbridge, Alberta T1J 3Z4Contact details
+1-403 382 55 00
[email protected]
-
Dr Kerstin Wernike
GERMANY
Address
Institute of Diagnostic Virology
Friedrich-Loeffler-Institut Federal Research Institute for Animal Health Südufer 10 17493 Greifswald – Insel Riems Insel RiemsContact details
+49-38351 7-1212
[email protected]
-
Dr Rebecca Strong
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-208 415 2102
[email protected]
Brucellosis (Brucella abortus)
-
Dra. Ana Maria Nicola
ARGENTINA
Address
National Service for Agri-Food Health and Quality (SENASA)- Argentina
Talcahuano 1660, Código Postal 1640, Martinez, Buenos AiresContact details
+54-11 48 74 67 31 (Ext. 2631 or 2730)
[email protected]
[email protected]
-
Dr Mahmoud Hamdy
EGYPT
Address
Department of Brucellosis Research
Animal Health Research Institute, Agricultural Research Center, Ministry of Agriculture and Land Reclamation, 7 Nadi El-Said Street, P.O. Box 12618, Dokki, GizaContact details
+201 222.28.14.76
[email protected]
[email protected]
-
Dr Claire Ponsart
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, F-94701 Maisons-Alfort CedexContact details
+33149771350
[email protected]
-
Dr. Heinrich Neubauer
GERMANY
Address
Institute of Bacterial Infections and Zoonoses, Friedrich-Loeffer Institute,
Naumburger Str. 96a, 07743 JenaContact details
+49-3641 804 2100
[email protected]
[email protected]
[email protected]
-
Dr. Svetlana Berdenstein
ISRAEL
Address
Kimron Veterinary Institute, Department of Bacteriology
P.O. Box 12, Beit Dagan 50250Contact details
+972-3 968 16 98
[email protected]
-
Dr Fabrizio De Massis
ITALY
Address
Istituto Zooprofilattico Sperimentaledell'Abruzzo e del Molise 'G. Caporale'
Via Campo Boario 64100 Teramo TERAMOContact details
+390-861 33 22 41
[email protected]
-
Dr Jin-Ju Lee
KOREA (REP. OF)
Address
Brucellosis Laboratory, Bacteriology DivisionAnimal and Plant Quarantine Agency (QIA)Ministry of Agriculture, Food and Rural Affairs (MAFRA)
177, Hyeoksin 8-ro, Gimcheon-si, Gyeongsangbuk-do, 39660 Gyeongsangbuk-doContact details
+82-54 912 0754
[email protected]
-
Prof. Liangquan Zhu
PEOPLE'S REPUBLIC OF CHINA
Contact details
+861062103630
[email protected]
[email protected]
[email protected]
-
Dr Monaya Ekgatat
THAILAND
Address
National Institute of Animal Health
50/2 Kasetklang Ladyao Chatuchak Bangkok 10900 THAILANDContact details
+66-2579 89.08 to 14 ext. 232
[email protected]
-
Prof. Ulrich Wernery
UNITED ARAB EMIRATES
Address
Central Veterinary Research Laboratory
P.O. Box 597 Dubai DubaiContact details
+971-4 337.51.65
[email protected]
-
Dr Adrian Whatmore
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35.76.10
[email protected]
Brucellosis (Brucella canis)
-
Prof. Ulrich Wernery
UNITED ARAB EMIRATES
Address
Central Veterinary Research Laboratory
P.O. Box 597 Dubai DubaiContact details
+971-4 337.51.65
[email protected]
Brucellosis (Brucella melitensis)
-
Dra. Ana Maria Nicola
ARGENTINA
Address
National Service for Agri-Food Health and Quality (SENASA)- Argentina
Talcahuano 1660, Código Postal 1640, Martinez, Buenos AiresContact details
+54-11 48 74 67 31 (Ext. 2631 or 2730)
[email protected]
[email protected]
-
Dr Mahmoud Hamdy
EGYPT
Address
Department of Brucellosis Research
Animal Health Research Institute, Agricultural Research Center, Ministry of Agriculture and Land Reclamation, 7 Nadi El-Said Street, P.O. Box 12618, Dokki, GizaContact details
+201 222.28.14.76
[email protected]
[email protected]
-
Dr Claire Ponsart
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, F-94701 Maisons-Alfort CedexContact details
+33149771350
[email protected]
-
Dr. Heinrich Neubauer
GERMANY
Address
Institute of Bacterial Infections and Zoonoses, Friedrich-Loeffer Institute,
Naumburger Str. 96a, 07743 JenaContact details
+49-3641 804 2100
[email protected]
[email protected]
[email protected]
-
Dr. Svetlana Berdenstein
ISRAEL
Address
Kimron Veterinary Institute, Department of Bacteriology
P.O. Box 12, Beit Dagan 50250Contact details
+972-3 968 16 98
[email protected]
-
Dr Fabrizio De Massis
ITALY
Address
Istituto Zooprofilattico Sperimentaledell'Abruzzo e del Molise 'G. Caporale'
Via Campo Boario 64100 Teramo TERAMOContact details
+390-861 33 22 41
[email protected]
-
Prof. Liangquan Zhu
PEOPLE'S REPUBLIC OF CHINA
Contact details
+861062103630
[email protected]
[email protected]
[email protected]
-
Dr Monaya Ekgatat
THAILAND
Address
National Institute of Animal Health
50/2 Kasetklang Ladyao Chatuchak Bangkok 10900 THAILANDContact details
+66-2579 89.08 to 14 ext. 232
[email protected]
-
Prof. Ulrich Wernery
UNITED ARAB EMIRATES
Address
Central Veterinary Research Laboratory
P.O. Box 597 Dubai DubaiContact details
+971-4 337.51.65
[email protected]
-
Dr Adrian Whatmore
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35.76.10
[email protected]
Brucellosis (Brucella suis)
-
Dra. Ana Maria Nicola
ARGENTINA
Address
National Service for Agri-Food Health and Quality (SENASA)- Argentina
Talcahuano 1660, Código Postal 1640, Martinez, Buenos AiresContact details
+54-11 48 74 67 31 (Ext. 2631 or 2730)
[email protected]
[email protected]
-
Dr Claire Ponsart
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, F-94701 Maisons-Alfort CedexContact details
+33149771350
[email protected]
-
Dr. Heinrich Neubauer
GERMANY
Address
Institute of Bacterial Infections and Zoonoses, Friedrich-Loeffer Institute,
Naumburger Str. 96a, 07743 JenaContact details
+49-3641 804 2100
[email protected]
[email protected]
[email protected]
-
Dr Fabrizio De Massis
ITALY
Address
Istituto Zooprofilattico Sperimentaledell'Abruzzo e del Molise 'G. Caporale'
Via Campo Boario 64100 Teramo TERAMOContact details
+390-861 33 22 41
[email protected]
-
Prof. Liangquan Zhu
PEOPLE'S REPUBLIC OF CHINA
Contact details
+861062103630
[email protected]
[email protected]
[email protected]
-
Dr Adrian Whatmore
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35.76.10
[email protected]
Camelpox
-
Prof. Ulrich Wernery
UNITED ARAB EMIRATES
Address
Central Veterinary Research Laboratory
P.O. Box 597 Dubai DubaiContact details
+971-4 337.51.65
[email protected]
Campylobacteriosis
-
Dr. Jaap Wagenaar
THE NETHERLANDS
Address
Department of Infectious Diseases and Immunology,
Faculty of Veterinary Medicine, trecht University, PO Box 80.165, 3508 TD UtrechtContact details
+31-320 23 81 57
[email protected]
Channel catfish virus disease
-
Dr Larry A. Hanson
UNITED STATES OF AMERICA
Address
Fish Diagnostic Laboratory, College of Veterinary Medicine, Mississippi State University
P.O. Box 6100, Spring Street, Mississippi 39762Contact details
+1-662 325 12 02
[email protected]
Chronic wasting disease
-
Dr. Gordon Mitchell
CANADA
Address
Canadian Food Inspection Agency, Ottawa Laboratory (Fallowfield), Animal Disease Research Institute
3851 Fallowfield Road, P.O. Box 11300, Station H, Nepean, Ontario K2H 8P9nContact details
+1-613 221.48.54
[email protected]
-
Dr Hyun-Joo Sohn
KOREA (REP. OF)
Address
Prion Disease Research Laboratory, Division of Foreign Animal DiseaseAnimal and Plant Quarantine Agency (QIA)Ministry of Agriculture, Food and Rural Affairs (MAFRA)
177, Hyeoksin 8-ro, Gimcheon-si, Gyeongsangbuk-do, 39660 Gyeongsangbuk-doContact details
+82-54 912 0862
[email protected]
-
Dr. Sylvie L. Benestad
NORWAY
Address
Norwegian Veterinary Institute (NVI)
Pb 64, N-1431 ÅsContact details
+47-23 21.60.00
[email protected]
[email protected]
-
Dr. Aaron Lehmkuhl
UNITED STATES OF AMERICA
Classical swine fever
-
Dr. Trevor Drew
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Geelong, Victoria 3220Contact details
+61 52.27.50.00
[email protected]
-
Dr Aruna Ambagala
CANADA
Address
National Centre for Foreign Animal Disease, Canadian Food Inspection Agency
1015 Arlington Street, Winnipeg, Manitoba R3E 3M4Contact details
+12047892089
[email protected]
[email protected]
-
Dr. Yu-Liang Huang
CHINESE TAIPEI
Address
Veterinary Research Institute, Council of Agriculture
376 Chung-Cheng Rd, Tamsui District, New Taipei City 25158Contact details
+886226212111
[email protected]
-
Prof. Paul Becher
GERMANY
Address
University of Veterinary Medicine of Hannover Department of Infectious DiseasesInstitute of Virology
Bünteweg 17 30559 Hannover HANOVREContact details
+49-511 953 88 40
[email protected]
-
Dr Katsuhiko Fukai
JAPAN
Address
National Institute of Animal HealthDepartment of Exotic Diseases
6-20-1 Josui-Honcho Kodaira Tokyo 187-0022 TOKYOContact details
+81-42 321 14 41
[email protected]
-
Prof. Qin Wang
PEOPLE'S REPUBLIC OF CHINA
Contact details
+86-010 612 55 400
[email protected]
[email protected]
[email protected]
-
Dr Katarzyna Podgórska
POLAND
Address
National Veterinary Research Institute
Partyzantow Str. 57 24-100 Pulawy PULAWYContact details
+48-81 889 30 47
[email protected]
[email protected]
-
Dra. Llilianne Ganges
SPAIN
-
Dr Helen Crooke
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-208 026 9665
[email protected]
Contagious agalactia
-
Dr Guido Loria
ITALY
Address
Istituto Zooprofilattico Sperimentale della Sicilia
Via G. Marinuzzi 3, 90129 PalermoContact details
+39-091 656 53 07
[email protected]
-
Dr Anne Ridley
UNITED KINGDOM
Address
Mycoplasma Group, Department of Statutory and Exotic Bacterial Diseases
Animal and Plant Health Agency, New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35 73 79
[email protected]
Contagious bovine pleuropneumonia
-
To Be Decided
BOTSWANA
Address
National Veterinary Laboratory
Private Bag 0035, GaboroneContact details
+267 392 87 16
[email protected]
-
Dr Lucia Manso-Silvan
FRANCE
Address
CIRAD Département BIOSUMR CIRAD-INRAe ASTRE : "Animal, Santé, Territoires, Risques, Ecosystèmes"
Campus International de Baillarguet TA A-117/E 34398 Montpellier Cedex 5 MONTPELLIERContact details
+33 (0)4 67 59 37 39
[email protected]
-
Dr Massimo Scacchia
ITALY
Address
Istituto Zooprofilattico Sperimentaledell'Abruzzo e del Molise 'G. Caporale'
Via Campo Boario 64100 Teramo TERAMOContact details
+390-861 33 24 05
[email protected]
-
Dr Ana Rosa Pombo Botelho
PORTUGAL
Address
Instituto Nacional de Investigação Agrária e Veterinária (INIAV, IP)
Av. da República, Quinta do Marquês, s/n 2780-157 Oeiras OEIRASContact details
+351-21 440 35 14
[email protected]
Contagious caprine pleuropneumonia
-
Dr Lucia Manso-Silvan
FRANCE
Address
CIRAD Département BIOSUMR CIRAD-INRAe ASTRE : "Animal, Santé, Territoires, Risques, Ecosystèmes"
Campus International de Baillarguet TA A-117/E 34398 Montpellier Cedex 5 MONTPELLIERContact details
+33 (0)4 67 59 37 39
[email protected]
-
Dr. Umit Özdemir
REPUBLIC OF TÜRKIYE
Address
Pandik Veterinary Control Institute
Batı mahallesi, Erol Kaya caddesi 1, 34890 İstanbulContact details
+90-216 390.12.80
[email protected]
Contagious equine metritis
-
Dr Sandrine Petry
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Normandy site), Physiopathology and Epidemiology of Equine Diseases Unit, 14430 DozuléContact details
+330231797971
[email protected]
-
Dr. Ian Mawhinney
UNITED KINGDOM
Address
Animal and Plant Health Agency Bury St Edmunds
Rougham Hill, Bury St Edmunds, Suffolk IP44 2RXContact details
+441284724499
[email protected]
-
Dr. Kristina Lantz
UNITED STATES OF AMERICA
Cysticercosis
-
Dr Xuenong Luo
PEOPLE'S REPUBLIC OF CHINA
Address
Department of Parasitology, State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute,
Chinese Academy of Agricultural Sciences, 1 Xujiaping, Lanzhou, Gansu Province 730046,Contact details
+86-931 832.39.78
[email protected]
Decapod iridescent virus 1 (DIV1)
-
Dr. Chien Tu
CHINESE TAIPEI
Dourine
-
Dr Laurent Hébert
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Normandy site), Physiopathology and Epidemiology of Equine Diseases Unit, 14430 DozuléContact details
+33-(0)2 31.79.22.76
[email protected]
Echinococcosis (Echinococcus granulosus and E. multilocularis)
-
Dr Giovanna Masala
ITALY
Address
National Reference Laboratory for Cyctic Echinococcosis (CE)
Istituto Zooprofilattico Sperimentale (IZS) of SardiniVia Duca degli Abruzzi, 807100 Sassari,, SassariContact details
+39-079 289 200
[email protected]
[email protected]
Enteric septicaemia of catfish (Edwardsiella ictaluri)
-
Dr Larry A. Hanson
UNITED STATES OF AMERICA
Address
Fish Diagnostic Laboratory, College of Veterinary Medicine, Mississippi State University
P.O. Box 6100, Spring Street, Mississippi 39762Contact details
+1-662 325 12 02
[email protected]
Enzootic abortion of ewes (ovine chlamydiosis)
-
Dr Karine Laroucau
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, 94701 Maisons-Alfort CedexContact details
+330149771350
[email protected]
-
Dr Christiane Schnee
GERMANY
Address
Institute of Molecular PathogenesisFriedrich-Loeffler-InstituteFederal Research Institute for Animal Health
Naumburger Str. 96a 07743 Jena JenaContact details
+49-3641 804 2435
[email protected]
-
Dr. Nicole Borel
SWITZERLAND
Address
Institute for Veterinary Pathology (IVPZ),
Vetsuisse Faculty, University of Zurich, Winterhurerstrasse 268, Zurich, CH-8057Contact details
+41446358571
[email protected]
Enzootic bovine leukosis
-
Dr Jacek Kuzmak
POLAND
Address
National Veterinary Research Institute
Al. Partyzantow str. 57 24-100 Pulawy PulawyContact details
+48-81 889.31.14
[email protected]
-
Dr Bhudipa Choudhury
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35.75.59
[email protected]
Epizootic haemorrhagic disease
-
Dr Stephan Zientara
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Virology unit, 14 rue Pierre et Marie Curie, 94701 Maisons-AlfortContact details
+33149771350
[email protected]
Equine infectious anaemia
-
Dr Maria Teresa Scicluna
ITALY
Address
Division for the Diagnosis of Viral Diseases and Leptospirosis
Istituto Zooprofillatico Sperimentale delle Regioni Lazio e Toscana (IZSLT) Via Appia Nuova 1411 00178 Rome RomeContact details
+390-6 79.09.93.15
[email protected]
-
Dr Xiaojun Wang
PEOPLE'S REPUBLIC OF CHINA
Address
Laboratory of Equine Infectious Anemia, Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences
427 Maduan Street, Harbin 150001Contact details
+86-189 46.06.60.85
[email protected]
Equine influenza
-
Prof. Ann Cullinane
IRELAND
Address
Irish Equine Centre
Johnstown, Naas, Co. KildareContact details
+353-45 86.62.66
[email protected]
-
Dr Manabu Nemoto
JAPAN
Address
Equine Research Institute, Japan Racing Association
1400-4 Shiba, Shimotsuke, Tochigi 329-0412Contact details
+81-285 44.0090
[email protected]
[email protected]
-
Dr Thomas M. Chambers
UNITED STATES OF AMERICA
Address
Department of Veterinary Science
Maxwell H. Gluck Equine Research Center, University of Kentucky, 108 Gluck Equine Research Center, Lexington, Kentucky 40546-0099Contact details
+1-859 257 47 57
[email protected]
Equine piroplasmosis
-
Prof. Naoaki Yokoyama
JAPAN
Address
Obihiro University of Agriculture and Veterinary Medicine Nishi 2-13, Inada-cho Obihiro, Hokkaido 080-8555 Hokkaido
Contact details
+81-155 49.56.49
[email protected]
Equine rhinopneumonitis
-
Prof. Ann Cullinane
IRELAND
Address
Irish Equine Centre
Johnstown, Naas, Co. KildareContact details
+353-45 86.62.66
[email protected]
-
Dr. Lutz Goehring
UNITED STATES OF AMERICA
Equine viral arteritis
-
Prof. Falko Steinbach
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35 75 66
[email protected]
-
To Be Decided
UNITED STATES OF AMERICA
Contact details
+1-859 257 47 57 ext. 81094
[email protected]
Escherichia coli
-
Dr John Morris Fairbrother
CANADA
Address
The Escherichia coli Laboratory (EcL)Faculty of Veternary Medicine, University of Montreal
3200 Sicotte Saint-Hyacinthe Québec J2S 2M2 QuébecContact details
+1-450 773.85.21
[email protected]
European foulbrood (infection of honey bees with Melissococcus plutonius)
-
Dr Marie-Pierre Chauzat
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia Antipolis Laboratory, Honey Bee Pathology Unit, Les Templiers 105 route des Chappes, CS 20111, 06902 Sophia AntipolisContact details
+33 (0)4 92 94 37 00
[email protected]
Foot and mouth disease
-
Dra. Sabrina Galdo
ARGENTINA
-
Dr. Joseph Hyera
BOTSWANA
-
Dr Edviges Maristela Pituco
BRAZIL
Address
PANAFTOSA
Av. President Kennedy 7778 25040-000 Duque de Caxias Rio de Janeiro Rio de JaneiroContact details
+55-21 36.61.90.64
[email protected]
-
Dr. Charles Nfon
CANADA
Address
National Centre for Foreign Animal Disease, Canadian Food Inspection Agency
Canadian Science Centre for Human and Animal Health, 1015 Arlington Street, Suite T2300, Winnipeg, Manitoba R3E 3M4Contact details
+1-204 789.20.23
[email protected]
-
Dr Bakkali Kassimi Labib
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Virology unit, 14 rue Pierre et Marie Curie, 94701 Maisons-Alfort CedexContact details
+330149771350
[email protected]
-
Santina Grazioli
ITALY
Address
Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (IZSLER)
Via A. Bianchi No. 9, 25124 BresciaContact details
+390-30 229 03 10
[email protected]
-
Dr. Sang-Ho Cha
KOREA (REP. OF)
Address
Division of Foot and Mouth Disease, Animal and Plant Quarantine Agency (QIA), Ministry of Agriculture, Food and Rural Affairs
177, Hyeoksin 8-ro, Gimcheon-si, Gyeongsangbuk-do, 39660Contact details
+82549120774
[email protected]
-
Dr Xiangtao Liu
PEOPLE'S REPUBLIC OF CHINA
Address
Lanzhou Veterinary Research Institute, CAAS, National Foot and Mouth Disease Reference Laboratory
Xujiaping No.1, Yanchangpu, Lanzhou, Gansu Province 730046Contact details
+86-931 834.25.85
[email protected]
[email protected]
-
Dr. Valery Zakharov
RUSSIA
Address
Federal Governmental Institute, Centre for Animal Health (FGI-ARRIAH)
600900 Yur'evets, VladmirContact details
+7-4922 26 06 14
[email protected]
[email protected]
-
Dr Livio Heath
SOUTH AFRICA
Address
Onderstepoort Veterinary Institute,
Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 95.01
[email protected]
-
Dr Donald King
UNITED KINGDOM
Address
Vesicular Disease Reference Laboratories
Ash Road, Pirbright Woking, Surrey, GU24 0NF PirbrightContact details
+44-1483 23.10.21
[email protected]
-
Vivian O'Donnell
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
40550 Route 25, Orient, New York, NY 11957Contact details
+16313233300
[email protected]
Glanders
-
Dr Karine Laroucau
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, 94701 Maisons-Alfort CedexContact details
+330149771350
[email protected]
-
Dr. Heinrich Neubauer
GERMANY
Address
Institute of Bacterial Infections and Zoonoses, Friedrich-Loeffer Institute,
Naumburger Str. 96a, 07743 JenaContact details
+49-3641 804 2100
[email protected]
[email protected]
[email protected]
-
Prof. Ulrich Wernery
UNITED ARAB EMIRATES
Address
Central Veterinary Research Laboratory
P.O. Box 597 Dubai DubaiContact details
+971-4 337.51.65
[email protected]
Heartwater
-
Dr Valérie Rodrigues
FRANCE
Address
UMR ASTRE : Animal, Santé, Territoires, Risques, Ecosystèmes ; CIRAD Département BIOS
Domaine Duclos – Prise-D'eau 97170 Petit-Bourg, GuadeloupeContact details
+33 (0)4 67 59 37 39
[email protected]
Hendra and Nipah virus diseases
-
Dr. Kim Halpin
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 00 00
[email protected]
[email protected]
Infection with Aphanomyces astaci (crayfish plague)
-
Dr Satu Viljamaa-Dirks
FINLAND
Address
Finnish Food Safety Authority
Ruokavirasto Kuopio Neulaniementie 4 70210 Kuopio KUOPIOContact details
+358 447.20.14.69
[email protected]
Infection with Bonamia exitiosa
-
Dr Isabelle Arzul
FRANCE
Address
IFREMER
Laboratoire de Génétique Aquaculture et Pathologie de Mollusques Marins 17390 La Tremblade La TrembladeContact details
+33 5 46.76.26.10
[email protected]
[email protected]
Infection with Bonamia ostreae
-
Dr Isabelle Arzul
FRANCE
Address
IFREMER
Laboratoire de Génétique Aquaculture et Pathologie de Mollusques Marins 17390 La Tremblade La TrembladeContact details
+33 5 46.76.26.10
[email protected]
[email protected]
Infection with Gyrodactylus salaris
-
Dr. Haakon Hansen
NORWAY
Address
Norwegian Veterinary Institute
Pb 64, N-1431 ÅsContact details
+47-23 21.61.23
[email protected]
[email protected]
Infection with Hepatobacter penaei (necrotising hepatopancreatitis)
-
Dr Arun Dhar
UNITED STATES OF AMERICA
Address
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E Lowell St, Building 90, 85721 TucsonContact details
+1-520 621 87.27
[email protected]
Infection with Macrobrachium rosenbergii nodavirus (white tail disease)
-
Dr A.Sait Sahul Hameed
INDIA
Address
C.Abdul Hakeem College Aquatic Animal Health Laboratory Department of Zoology
Melvisharam-632 509 Ranipet District, Tamil Nadu Tamil NaduContact details
+91-4172 26.94.87
[email protected]
Infection with Marteilia refringens
-
Dr Isabelle Arzul
FRANCE
Address
IFREMER
Laboratoire de Génétique Aquaculture et Pathologie de Mollusques Marins 17390 La Tremblade La TrembladeContact details
+33 5 46.76.26.10
[email protected]
[email protected]
Infection with Marteilia sydneyi
-
Dr Isabelle Arzul
FRANCE
Address
IFREMER
Laboratoire de Génétique Aquaculture et Pathologie de Mollusques Marins 17390 La Tremblade La TrembladeContact details
+33 5 46.76.26.10
[email protected]
[email protected]
Infection with Mikrocytos mackini
-
Dr Cathryn Abbot
CANADA
Address
Department of Fisheries and Oceans, Pacific Biological Station
3190 Hammond Bay Road, Nanaimo, British Colombia V9T 6N7Contact details
+12506186166
[email protected]
Infection with Taura syndrome virus
-
Dr Arun Dhar
UNITED STATES OF AMERICA
Address
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E Lowell St, Building 90, 85721 TucsonContact details
+1-520 621 87.27
[email protected]
Infection with abalone herpesvirus
-
Dr Nick Moody
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 00 00
[email protected]
Infection with epizootic haematopoietic necrosis virus
-
Dr Nick Moody
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 00 00
[email protected]
Infection with infectious haematopoietic necrosis virus
-
Dr Kyle Garver
CANADA
Address
Pacific Biological Station – Aquatic Animal Health Laboratory (PBS-AAHL)
Fisheries & Oceans Canada, 3190 Hammond Bay Road, Nanaimo V9T 6N7, British ColumbiaContact details
+1-250 756 73 40
[email protected]
-
Dr. Hong Liu
PEOPLE'S REPUBLIC OF CHINA
Address
Animal and Plant Inspection and Quarantine Technical Center, Shenzhen Customs District
General Administration of Customs, P. R. China (GACC), Building 1011 of Fuqiang Road, Futianqu, Shenzhen, Guangdong Province, 518045Contact details
+86-755 25 58 84 10
[email protected]
[email protected]
Infection with infectious hypodermal and haematopoietic necrosis virus
-
Dr Bing Yang
PEOPLE'S REPUBLIC OF CHINA
Address
Maricultural Organism Disease Control and Molecular Pathology Laboratory, Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences, 106 Nanjing Road, Qingdao, Shandong 266071Contact details
+86 532 858 230 62
[email protected]
-
Dr Arun Dhar
UNITED STATES OF AMERICA
Address
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E Lowell St, Building 90, 85721 TucsonContact details
+1-520 621 87.27
[email protected]
Infection with infectious salmon anaemia virus
-
Dr. Sergio Hernán Marshall González
CHILE
Address
Aquaculture Pathology Laboratory,
Pontifical Catholic University of Valparaíso, Curauma Campus, Avenida Universidad 330, ValparaísoContact details
+56322274828
[email protected]
[email protected]
-
Dr. Ole Bendik Dale
NORWAY
Address
Norwegian Veterinary Institute
Pb 64, N-1431 ÅsContact details
+47-23 21 60 00
[email protected]
[email protected]
Infection with koi herpesvirus
-
Dr. Heike Schutze
GERMANY
Address
German Reference Laboratory for Koi Herpesvirus Disease (KHVD)
Institute of Infectology (IMED), Friedrich-Loeffler-Institut (FLI), Federal Research Institute for Animal Health, Institute of Infectology, Südufer 10, 17493 Greifswald – Insel RiemsContact details
+493835171254
[email protected]
-
Dr Takafumi Ito
JAPAN
Address
Aquaculture Research Department, Fisheries Technology Institute,
Japan Fisheries Research and Education Agency, Nakatsuhamaura 422-1, Minami-ise, Mie 516-0193Contact details
+81599661872
[email protected]
-
Dr. Irene Cano Cejas
UNITED KINGDOM
Address
The Centre for Environment, Fisheries and Aquaculture Science (CEFAS)Weymouth Laboratory
Barrack Road, The Nothe, Weymouth, Dorset DT4 8UB WeymouthContact details
+44-1305 20.66.42
[email protected]
Infection with ranavirus
-
Dr Nick Moody
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 00 00
[email protected]
Infection with red sea bream iridovirus
-
Dr Yasuhiko Kawato
JAPAN
Address
National Research Institute of Aquaculture Fisheries Research Agency
422-1 Nakatsuhamaura Minami-ise, Mie 516-0193 MieContact details
+81-599 66.18.30
[email protected]
Infection with salmonid alphavirus
-
Dr. Hilde Sindre
NORWAY
Address
Norwegian Veterinary Institute
Pb 64, N-1431 ÅsContact details
+47 23.21.60.00
[email protected]
[email protected]
Infection with spring viraemia of carp virus
-
Dr. Hong Liu
PEOPLE'S REPUBLIC OF CHINA
Address
Animal and Plant Inspection and Quarantine Technical Center, Shenzhen Customs District
General Administration of Customs, P. R. China (GACC), Building 1011 of Fuqiang Road, Futianqu, Shenzhen, Guangdong Province, 518045Contact details
+86-755 25 58 84 10
[email protected]
[email protected]
-
Dr. Richard Paley
UNITED KINGDOM
Contact details
+441305206642
[email protected]
Infection with viral haemorrhagic septicaemia virus
-
Dr Kyle Garver
CANADA
Address
Pacific Biological Station – Aquatic Animal Health Laboratory (PBS-AAHL)
Fisheries & Oceans Canada, 3190 Hammond Bay Road, Nanaimo V9T 6N7, British ColumbiaContact details
+1-250 756 73 40
[email protected]
-
Dr Britt Bang Jansen
DENMARK
Address
National Institute for Aquatic Resources
Kemitorvet, Building 202 2800 Kgs, Lyngby LyngbyContact details
+45 35 88 68 31
[email protected]
-
Dr Hyoung Jun Kim
KOREA (REP. OF)
Address
Pathology Research Division in Aquaculture Research Department
National Institute of Fisheries Science (NIFS), Ministry of Oceans and Fisheries, 216 Gijanghaean-ro, Gijang-eup, Busan, 46082Contact details
+82-51 720.2114
[email protected]
Infection with white spot syndrome virus
-
Prof. Han-Ching Wang
CHINESE TAIPEI
Address
International Center for the Scientific Development of Shrimp Aquaculture (ICDSA), National Cheng Kung University (NCKU)
No. 500, Sec. 3, Anming Road, Annan District, Tainan City 709Contact details
+886-6 384 24 48
[email protected]
-
Dr Qingli Zhang
PEOPLE'S REPUBLIC OF CHINA
Address
Maricultural Organism Disease Control and Molecular Pathology Laboratory Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences 106 Nanjing Road, Qingdao Shandong 266071 CHINA (PEOPLE'S REP. OF)Contact details
+86 532 858 230 62 ext 812
[email protected]
-
Dr Arun Dhar
UNITED STATES OF AMERICA
Address
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E Lowell St, Building 90, 85721 TucsonContact details
+1-520 621 87.27
[email protected]
Infection with yellow head virus genotype 1
-
Dr Nick Moody
AUSTRALIA
Address
CSIRO Australian Centre for Disease Preparedness
5 Portarlington Road, Private Bag 24 (Ryrie Street), Geelong 3220, VictoriaContact details
+61-3 52 27 00 00
[email protected]
Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis
-
Dr. Martin Beer
GERMANY
Address
Institute of Diagnostic VirologyFriedrich-Loeffler Institut,Federal Research Institute for Animal Health
Südufer 10 D-17493 Greifswald, Insel RiemsContact details
+49 38351 7 1223
[email protected]
[email protected]
-
Dr Akbar Dastjerdi
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35.75.08
[email protected]
Infectious bursal disease (Gumboro disease)
-
Dr Nicolas Eterradossi
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Ploufragan-Plouzané-Niort Laboratory, Poultry and Rabbit Virology, Immunology, Parasitology Unit, B.P. 53, 22440 PloufraganContact details
+33 (0)2 96 01 62 22
[email protected]
-
Dr. Yulong Gao
PEOPLE'S REPUBLIC OF CHINA
Address
Division of Avian Immunosuppressive Disease, Harbin Veterinary Research Institute (HVRI)
Chinese Academy of Agricultural Sciences (CAAS), 678 Haping Road, Xiangfang District, Harbin 150069Contact details
+8618945083045864
[email protected]
Infestation of honey bees with Aethina tumida (small hive beetle)
-
Dr Stéphanie Franco
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia Antipolis Laboratory, Honey Bee Pathology Unit, Les Templiers 105 route des Chappes, CS 20111, 06902 Sophia AntipolisContact details
+33 (0)4 92 94 37 00
[email protected]
-
Dr Marc O. Schäfer
GERMANY
Address
National Reference Laboratory for Bee Diseases, Friedrich-Loeffer-Institut
Federal Research Institute for Animal Health, Institute of Infectology, Südufer 1017493 Greifswald – Insel RiemsContact details
+49-38351 7 1246
[email protected]
Infestation of honey bees with Tropilaelaps spp.
-
Dr Stéphanie Franco
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia Antipolis Laboratory, Honey Bee Pathology Unit, Les Templiers 105 route des Chappes, CS 20111, 06902 Sophia AntipolisContact details
+33 (0)4 92 94 37 00
[email protected]
Japanese encephalitis
-
Dr Dong-Kun Yang
KOREA (REP. OF)
Address
Animal and Plant Quarantine Agency
177 Hyeoksin 8-ro Gimcheong-si Gyeongsangbuk-do 39660 GyeongsangbukContact details
+82 31 467.1783
[email protected]
Leishmaniosis
-
Dr Fabrizio Vitale
ITALY
Address
Dept Molecular Biology
Istituto Zooprofilattico Sperimentale della Sicilia (IZSSi), National Reference Centre for Leishmaniasis (C.Re.Na.L.), via Gino Marinuzzi 3, 90129, Palermo PalermoContact details
39-091 656.53.68
[email protected]
Leptospirosis
-
Dra. Jessica Petrakovsky
ARGENTINA
Address
Laboratorio de Leptospirosis, Dirección General de Laboratorios y Control Técnico, Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA)
Avenida Talcahuano N° 1660 (1640), Martínez, Pcia de Buenos AiresContact details
+54-11 48.36.11.14 int 287 o 299
[email protected]
-
Dr. Luis Samartino
ARGENTINA
Address
CICVyA INTA, Instituto de Bacteriología
Casilla de Correo 25, Castelar 1712, Moron, Provincia de Buenos AiresContact details
+541146211289
[email protected]
-
Dr. Paula Ristow
THE NETHERLANDS
Address
Academic Medical Center, Department of Medical Microbiology and Infection Prevention
University of Amsterdam, Meibergdreef 39, 1105 AZ AmsterdamContact details
+31205665431
[email protected]
-
Dr. Matthew Erdman
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
1920 Dayton Avenue, Ames, Iowa 50010Contact details
+15153377200
[email protected]
[email protected]
[email protected]
Lumpy skin disease
-
Dr. Nick De Regge
BELGIUM
Contact details
+3223790514
[email protected]
-
Dr. Antoinette Van Schalkwyk
SOUTH AFRICA
Address
Senior Researcher
Onderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort 0110,Contact details
+27125299108
[email protected]
-
Georgina Limon-Vega
UNITED KINGDOM
Address
Pirbright,
Ash Road, Woking, Surrey GU24 0NFContact details
+44-1483 23.24.41
[email protected]
Mammalian tuberculosis
-
Dr Bernardo Alonso
ARGENTINA
Address
DILAB (Dirección de Laboratorios y Control Técnico) Servicio Nacional de Sanidad y Calidad, Agroalimentaria (SENASA)
Talcahuano 1660,1640 Martínez, Prov. de Buenos AiresContact details
+54-11 48.74.67.35
[email protected]
[email protected]
-
Dr María Laura Boschiroli-Cara
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, 94701 Maisons-Alfort CedexContact details
+330149771350
[email protected]
-
Dra. Beatriz ROMERO MARTINEZ
SPAIN
Contact details
+34913944033
[email protected]
[email protected]
-
Dr Jason Sawyer
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 34.11.11
[email protected]
-
Dr. Tyler C. Thacker
UNITED STATES OF AMERICA
Marek's disease
-
Dr. Yongxiu Yao
UNITED KINGDOM
Middle East respiratory syndrome
-
Prof. Ulrich Wernery
UNITED ARAB EMIRATES
Address
Central Veterinary Research Laboratory
P.O. Box 597 Dubai DubaiContact details
+971-4 337.51.65
[email protected]
Myxomatosis
-
Dr. Antonio Lavazza
ITALY
Address
Dept. Animal Health and Welfare
Via Antonio Bianchi 9, 25124 BresciaContact details
+39-030 22.90.617
[email protected]
Nagana: infections with salivarian trypanosomes
-
Dr Marc Desquesnes
FRANCE
Address
UMR177-Intertryp (CIRAD-IRD)CIRAD-bios
Campus international de Baillarguet TA A-17 / G 34398 Montpellier Cedex 5 MontpellierContact details
+33-(0)4 67 59 37 24
[email protected]
New world screwworm (Cochliomyia hominivorax)
-
Dr. John B. Welch
PANAMA
Newcastle disease
-
Dr Dilmara Reischak
BRAZIL
Address
Laboratório Federal de Defesa Agropecuária em Sao Paulo – LFDA-SPUnidade de Sanidade Aviária
Rua Raul Ferrari, s/n° Jardim Santa Marcelina CEP 13100-105 Campinas SP Sao PauloContact details
+55-19 32.52.31.74
[email protected]
-
Dr Christian Grund
GERMANY
Address
Institute of Diagnostic Virology, Friedrich-Loeffler-Institute
Federal Research Institute for Animal Health, Südufer 10, D-17493 Greifswald, Insel RiemsContact details
+49-38351 7 1196
[email protected]
-
Dr Isabella Monne
ITALY
Address
Istituto Zooprofilattico Sperimentale delle Venezie, Research and Innovation Dept.
Viale Dell'Università 10, 35020 Legnaro PDContact details
+39-049 808 4381
[email protected]
-
Dr Ji-Ye Kim
KOREA (REP. OF)
Address
Newcastle disease Laboratory, Avian Disease division, Animal and Plant Quarantine Agency (QIA)
Ministry of Agriculture, Food and Rural Affairs (MAFRA), 177, Hyeoksin 8-ro, Gimcheon-si, Gyeongsangbuk-do, 39660Contact details
+82-54 912 0968
[email protected]
-
Dr Zhiliang Wang
PEOPLE'S REPUBLIC OF CHINA
Address
National Surveillance and Research Center for Exotic Animal Diseases
China Animal Health and Epidemiology Center, 369 Nanjing Road, Qingdao 266032Contact details
+86-532 85.63.91.66
[email protected]
[email protected]
-
Dr. Viktor N. Irza
RUSSIA
Address
National Reference Laboratory for Avian Influenza and Newcastle Disease
Federal State-Financed Institution “Federal Centre for Animal Health” (FGBI “ARRIAH”), Yur'evets, Vladimir 60090Contact details
+7-4922 26 18 67
[email protected]
[email protected]
-
To Be Decided
UNITED KINGDOM
Address
WOAH/FAO International Reference Laboratory for Avian Influenza
Animal and Plant Health Agency – Weybridge, Addlestone, Surrey KT15 3NBContact details
+441483232441
[email protected]
[email protected]
-
Dr. Mia Kim Torchetti
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
1920 Dayton Avenue, Ames, Iowa 50010Contact details
+15153377551
[email protected]
Nosemosis of honey bees
-
Dr Marie-Pierre Chauzat
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia Antipolis Laboratory, Honey Bee Pathology Unit, Les Templiers 105 route des Chappes, CS 20111, 06902 Sophia AntipolisContact details
+33 (0)4 92 94 37 00
[email protected]
Oncorhynchus masou virus disease
-
Dr Hisae Kasai
JAPAN
Address
Laboratory of Biotechnology and Microbiology Faculty of Fisheries Science Hokkaido University
3-1-1 Minato-Cho Hakodate Hokkaido 041-8611 HOKKAIDOContact details
+81-138 40 88 98
[email protected]
Ovine epididymitis (Brucella ovis)
-
Dra. Ana Maria Nicola
ARGENTINA
Address
National Service for Agri-Food Health and Quality (SENASA)- Argentina
Talcahuano 1660, Código Postal 1640, Martinez, Buenos AiresContact details
+54-11 48 74 67 31 (Ext. 2631 or 2730)
[email protected]
[email protected]
-
Dr Claire Ponsart
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Animal Health Laboratory (Maisons-Alfort), Bacterial Zoonoses Unit, 14 rue Pierre et Marie Curie, F-94701 Maisons-Alfort CedexContact details
+33149771350
[email protected]
-
Dr Fabrizio De Massis
ITALY
Address
Istituto Zooprofilattico Sperimentaledell'Abruzzo e del Molise 'G. Caporale'
Via Campo Boario 64100 Teramo TERAMOContact details
+390-861 33 22 41
[email protected]
-
Dr Adrian Whatmore
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35.76.10
[email protected]
Ovine theileriosis
-
Prof. Hong Yin
PEOPLE'S REPUBLIC OF CHINA
Address
Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences(CAAS)
Vector and Vector and Vector-borne Diseases Control Laboratory (VVBDC), Xujiaping 1, Chengguan District, Lanzhou, Gansu Province 730046Contact details
+86-93 18.34.26.81
[email protected]
Paratuberculosis
-
Dr Bernardo Alonso
ARGENTINA
Address
DILAB (Dirección de Laboratorios y Control Técnico) Servicio Nacional de Sanidad y Calidad, Agroalimentaria (SENASA)
Talcahuano 1660,1640 Martínez, Prov. de Buenos AiresContact details
+54-11 48.74.67.35
[email protected]
[email protected]
-
Dr Virginie Poisson
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Ploufragan-Plouzané- Niort Laboratory, 60 rue de Pied-de-Fond, CS 28440, 79024 Niort CedexContact details
+33 (0)5 49.79.61.28
[email protected]
-
Dr. Matteo Ricchi
ITALY
Peste des petits ruminants
-
Dr. Arnaud Bataille
FRANCE
Address
CIRAD-Bios
Campus International de Baillarguet TA A-15/G, 34398 Montpellier Cedex 5Contact details
+33 (0)4 67 59 37 98
[email protected]
-
Dr Zhiliang Wang
PEOPLE'S REPUBLIC OF CHINA
Address
National Surveillance and Research Center for Exotic Animal Diseases
China Animal Health and Epidemiology Center, 369 Nanjing Road, Qingdao 266032Contact details
+86-532 85.63.91.66
[email protected]
[email protected]
-
Dr Michael Baron
UNITED KINGDOM
Address
Ash Road, Pirbright Woking, Surrey, GU24 0NFContact details
+44-1483 23.24.41
[email protected]
Porcine reproductive and respiratory syndrome
-
Dr Kegong Tian
PEOPLE'S REPUBLIC OF CHINA
Address
Veterinary Diagnostic LaboratoryChina Animal Disease Control Center
No.17 Tiangui Street Biomedical Base Daxing District Beijing 102618 BeijingContact details
+86-10 59.19.88.96
[email protected]
[email protected]
-
Dr Katarzyna Podgórska
POLAND
Address
National Veterinary Research Institute
Partyzantow Str. 57 24-100 Pulawy PULAWYContact details
+48-81 889 30 47
[email protected]
[email protected]
Q fever
-
Dr Elodie Rousset
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia-Antipolis Laboratory, Animal Q fever, 105, route des Chappes, BP 111, 06902 Sophia-AntipolisContact details
+33-4 92.94.37.00
[email protected]
-
Dr. Agnieszka Jodelko
POLAND
Address
National Veterinary Research Institute, Department of Cattle and Sheep Diseases
Al. Partyzantow str. 5724-100 PulawyContact details
+48818893274
[email protected]
[email protected]
Rabbit haemorrhagic disease
-
Dr. Patrizia Cavadini
ITALY
Address
Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna 'B. Ubertini'
Via A. Bianchi No. 7/9, 25124 Brescia,Contact details
+39-30 229 06 17
[email protected]
Rabies
-
Dr. Christine Fehlner-Gardiner
CANADA
Address
Centre of Expertise for Rabies CFIA/ACIA, Ottawa Laboratory Fallowfield, Animal Diseases Research Institute
3851 Fallowfield Road, P.O. Box 11300, Station H, Nepean, Ontario K2H 8P9Contact details
+1-343 212 03 04
[email protected]
-
Dr Florence Cliquet
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Laboratory for Rabies and Wildlife (Nancy), Technopôle agricole et vétérinaire Domaine de Pixérécourt, B.P. 40009, 54220 Malzéville CedexContact details
+33 (0)3 83 29 89 50
[email protected]
-
Dr Thomas Müller
GERMANY
Address
Institute of Molecular Virology and Cell Biology, Friedrich-Loeffler Institut,Federal Research Institute for Animal Health
Südufer 10 D-17493 Greifswald – Insel Riems GreifswaldContact details
+49-38351 7 1659
[email protected]
-
Dr Shrikrishna Isloor
INDIA
Address
OIE Twinned KVAFSU-CVA Rabies Diagnostic Laboratory, Department of Microbiology, Veterinary College
Karnataka Veterinary, Animal and Fisheries Sciences University (KVAFSU), Hebbal, Bangalore 560024,Contact details
+91-80 29.53.22.87
[email protected]
-
Dr Boris Yakobson
ISRAEL
Address
Kimron Veterinary Institute
Veterinary Services and Animal Health, P.O. Box 12, Beit Dagan 50250Contact details
+ 972-3 9681720
[email protected]
-
Dr Dong-Kun Yang
KOREA (REP. OF)
Address
Animal and Plant Quarantine Agency
177 Hyeoksin 8-ro Gimcheong-si Gyeongsangbuk-do 39660 GyeongsangbukContact details
+82 31 467.1783
[email protected]
-
Dr. Juan Antonio Montaño Hirose
MEXICO
Address
Centro Nacional de Servicios de Diagnóstico en Salud Animal
Av. Centenario de la Educación s/n (Km 37.5 Carretera Federal México – Pachuca) 55740 Tecámac de Felipe Villanueva Tecámac, Estado de México TecámacContact details
+525559051000
[email protected]
[email protected]
-
Prof. Changchun Tu
PEOPLE'S REPUBLIC OF CHINA
-
Dr Vlad Vuta
ROMANIA
Address
National Reference Laboratory for Rabies, Institute for Diagnosis and Animal Health
Dr Nicolae Staicovici Street, No. 63, Sector 5, Bucharest 050557Contact details
+40-374 32.20.00
[email protected]
[email protected]
-
Dr Claude Taurai Sabeta
SOUTH AFRICA
Address
Rabies Unit
Onderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 94 39
[email protected]
-
Dr Anthony Fooks
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-208 415.22.38
[email protected]
-
Dr. Ryan Wallace
UNITED STATES OF AMERICA
Address
Poxvirus and Rabies Branch, Division of High-Consequence Pathogens and Pathology
National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, 1600 Clifton Road, NE, Mail Stop G33, Atlanta, GA 30 333Contact details
+1-404 639.10.50
[email protected]
Rift Valley fever
-
Dre. Catherine Cetre-Sossah
FRANCE
-
Dr Baratang Alison Lubisi
SOUTH AFRICA
Address
Senior Research Veterinarian
Onderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 91 17
[email protected]
Rinderpest
-
Dr. Arnaud Bataille
FRANCE
Address
CIRAD-Bios
Campus International de Baillarguet TA A-15/G, 34398 Montpellier Cedex 5Contact details
+33 (0)4 67 59 37 98
[email protected]
-
Dr Takehiro Kokuho
JAPAN
Address
Exotic Disease Research DivisionNational Institute of Animal Health (NIAH)
National Agriculture and Food Research Organization (NARO) Josuihoncho 6-20-1 Kodaira Tokyo, 187-0022 TokyoContact details
+81-42 321.14 66
[email protected]
-
Dr Michael Baron
UNITED KINGDOM
Address
Ash Road, Pirbright Woking, Surrey, GU24 0NFContact details
+44-1483 23.24.41
[email protected]
-
Dr. Wei Jia
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
40550 Route 25, Orient, New York, NY 11957Contact details
+16313233197
[email protected]
Salmonellosis
-
Dr. Gitanjali Arya
CANADA
Address
The Guelph Reference Services Unit, The National Microbiology Laboratory at Guelph
Public Health Agency of Canada, 110 Stone Road West, Guelph, ON N1G 3W4Contact details
+1-519 826.26.40
[email protected]
-
Dr Istvan Szabo
GERMANY
Address
Federal Institute for Risk Assessment
Diedersdorfer Weg 1 D-12277 Berlin BERLINContact details
+49-30 184 12 22 20
[email protected]
-
Dr. Antonia Ricci
ITALY
Address
Istituto Zooprofilattico Sperimentale delle Venezie, National Reference Laboratory for Salmonella
Viale dell'Università, 10,35020 Legnaro (Padova), LegnaroContact details
+39-049 8084.296
[email protected]
-
Dr Min-Su Kang
KOREA (REP. OF)
Address
Avian Bacteriology LaboratoryAvian Disease Research Division
Animal and Plant Quarantine Agency (APQA) Ministry of Agriculture, Food and Rural Affairs (MAFRA) 177, Hyeoksin 8-ro Gimcheon-si Gyeongsangbuk-do, 39660, Gimcheon-siContact details
+82-54 912 08 18
[email protected]
-
Dr. Francesca Martelli
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44-1932 35 73 61
[email protected]
Scrapie
-
Dr. Gordon Mitchell
CANADA
Address
Canadian Food Inspection Agency, Ottawa Laboratory (Fallowfield), Animal Disease Research Institute
3851 Fallowfield Road, P.O. Box 11300, Station H, Nepean, Ontario K2H 8P9nContact details
+1-613 221.48.54
[email protected]
-
Dr. Cristina Casalone
ITALY
Address
Italian National Reference Centre for Diagnostic Activities in Stranded Marine Mammals (C.Re.Di.Ma.), via Bologna 148, 10154 TorinoContact details
+39-11 26 86 296
[email protected]
[email protected]
-
Dr Juan José Badiola Díez
SPAIN
Address
Centro de investigación en Encefalopatías y enfermedades transmisibles emergentes
Universidad de Zaragoza, Facultad de Veterinaria, Departamento de Patología Animal, Miguel Servet 177, 50013 ZaragozaContact details
+34-976 76 20 19
[email protected]
-
Prof. Torsten Seuberlich
SWITZERLAND
Address
Neuro Centre Department of Clinical Research and Veterinary Public Health
Division of Experimental Clinical Research, University of Bern, Bremgartenstrasse 109 A3012 Bern,Contact details
+41-31 631 22 06
[email protected]
-
Dr John Spiropoulos
UNITED KINGDOM
Address
Animal and Plant Health Agency
New Haw, Addlestone, Weybridge, Surrey KT15 3NBContact details
+44 1932 357.564
[email protected]
Sheep pox and goat pox
-
Dr. Mohammad Hassan Ebrahimi-jam
IRAN
Address
RAZI Vaccine & Serum Research Institute
Karaj 3197619751, Teheran,Contact details
+982634570038
[email protected]
[email protected]
-
Dr Baratang Alison Lubisi
SOUTH AFRICA
Address
Senior Research Veterinarian
Onderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort 0110Contact details
+27-12 529 91 17
[email protected]
-
Georgina Limon-Vega
UNITED KINGDOM
Address
Pirbright,
Ash Road, Woking, Surrey GU24 0NFContact details
+44-1483 23.24.41
[email protected]
Surra (Trypanosoma evansi)
-
Dr. Nick Van Reet
BELGIUM
Address
Institute of Tropical Medicine AntwerpDepartment of Parasitology
Nationalestraat 155B-2000 AntwerpenContact details
+32-3 247.63.71
[email protected]
-
Prof. Noboru Inoue
JAPAN
Address
National Research Center for Protozoan DiseasesObihiro University of Agricultureand Veterinary Medicine
Inada-cho Nishi 2-13 Obihiro, Hokkaido 080-8555 HokkaidoContact details
+81-155 49.56.47
[email protected]
Swine influenza
-
Dr Chiara Chiapponi
ITALY
-
Dr. Junki Mine
JAPAN
Address
Viral Disease and Epidemiology Research Division, National Institute of Animal Health, National Agriculture and Food Research Organization
Kannondai, Tsukuba, Ibaraki, 305-0856Contact details
+81298387704
[email protected]
-
To Be Decided
UNITED KINGDOM
Address
WOAH/FAO International Reference Laboratory for Avian Influenza
Animal and Plant Health Agency – Weybridge, Addlestone, Surrey KT15 3NBContact details
+441483232441
[email protected]
[email protected]
-
Dr. Mia Kim Torchetti
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
1920 Dayton Avenue, Ames, Iowa 50010Contact details
+15153377551
[email protected]
Swine streptococcosis
-
Prof. Chengping Lu
PEOPLE'S REPUBLIC OF CHINA
Address
Nanjing Agricultural University (NAU)
Branch of Swine Streptococcosis Diagnostic Laboratory (BSSDL), Weigang No.1, Nanjing, Jiangsu ProvinceContact details
+86-25 84.39.53.28
[email protected]
[email protected]
Swine vesicular disease
-
Giulia Pezzoni
ITALY
Address
Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (IZSLER)
Via A. Bianchi No. 9, 25124 BresciaContact details
+390302290310
[email protected]
-
Dr Donald King
UNITED KINGDOM
Address
Vesicular Disease Reference Laboratories
Ash Road, Pirbright Woking, Surrey, GU24 0NF PirbrightContact details
+44-1483 23.10.21
[email protected]
Theileriosis
-
To Be Decided
ITALY
Address
Istituto Zooprofilattico Sperimentale della Sicilia (IZSSi),
Italian Reference Centre for Anaplasma, Babesia, Rickettsia, Theileria (C.R.A.Ba.R.T.), via Gino Marinuzzi 3, 90129, PalermoContact details
+39-091 656.53.41 ext 219
[email protected]
Trichinellosis
-
Dr Brad Scandrett
CANADA
Address
Canadian Food Inspection Agency 116 Veterinary Road Saskatoon Saskatchewan S7N 2R3 Saskatoon
Contact details
+1-306 385.78.18
[email protected]
-
Dr. Maria Angeles Gomez Morales
ITALY
Address
Istituto Superiore di Sanita, Laboratorio di Parasitoligia
Viale Regina Elena 299, 00161 RomaContact details
+390-6 49.90.23.04
[email protected]
Trypanosomosis (tsetse-transmitted)
-
Dr Marc Desquesnes
FRANCE
Address
UMR177-Intertryp (CIRAD-IRD)CIRAD-bios
Campus international de Baillarguet TA A-17 / G 34398 Montpellier Cedex 5 MontpellierContact details
+33-(0)4 67 59 37 24
[email protected]
Turkey rhinotracheitis
-
Dr Nicolas Eterradossi
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Ploufragan-Plouzané-Niort Laboratory, Poultry and Rabbit Virology, Immunology, Parasitology Unit, B.P. 53, 22440 PloufraganContact details
+33 (0)2 96 01 62 22
[email protected]
Varroosis (infestation of honey bees with Varroa spp.)
-
Dr Marie-Pierre Chauzat
FRANCE
Address
ANSES (French Agency for Food, Environmental and Occupational Health & Safety)
Sophia Antipolis Laboratory, Honey Bee Pathology Unit, Les Templiers 105 route des Chappes, CS 20111, 06902 Sophia AntipolisContact details
+33 (0)4 92 94 37 00
[email protected]
-
Dr Marc O. Schäfer
GERMANY
Address
National Reference Laboratory for Bee Diseases, Friedrich-Loeffer-Institut
Federal Research Institute for Animal Health, Institute of Infectology, Südufer 1017493 Greifswald – Insel RiemsContact details
+49-38351 7 1246
[email protected]
-
Dr. Richard J. Hall
NEW ZEALAND
Contact details
+6448945600
[email protected]
Vesicular stomatitis
-
Dr Edviges Maristela Pituco
BRAZIL
Address
PANAFTOSA
Av. President Kennedy 7778 25040-000 Duque de Caxias Rio de Janeiro Rio de JaneiroContact details
+55-21 36.61.90.64
[email protected]
-
Dr. Rachel Tell
UNITED STATES OF AMERICA
Address
National Veterinary Services Laboratories, USDA, APHIS, VS
1920 Dayton Avenue, Ames, Iowa 50010Contact details
+15153377551
[email protected]
Viral encephalopathy and retinopathy
-
Dr Anna Toffan
ITALY
Address
Istituto Zooprofilattico Sperimentale delle Venezie, Fish Virology Dept
Viale dell'Università, 10,35020 Legnaro (Padova)Contact details
+39-049 808 43 88
[email protected]
West Nile Fever
-
Dr Federica Monaco
ITALY
Address
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale"
Via Campo Boario, 64100 TeramoContact details
+39 0861 33.22.05
[email protected]
1. Introduction
1.1. Purpose
This document provides guidelines for evaluation of veterinary laboratory capability to conduct diagnostic tests for infectious diseases.
1.2. Scope
These guidelines are intended for use by WOAH Member Countries as part of the evaluation of laboratories that are carrying out tests to qualify animals and animal products for international movement. These guidelines should be used in conjunction with the WOAH Guidelines for Laboratory Quality Evaluation for overall assessment of laboratory quality and capability.
This guide is based on the relevant requirements of the ISO[1] 9000 series of standards, ISO/IEC[2] 17025 and ISO Guide 43.
1.3. Interlaboratory test comparisons
Interlaboratory test comparisons may be undertaken for a variety of reasons which may include:
i) Determining a laboratory’s capability to conduct specific diagnostic tests,
ii) Checking or certifying the performance of individual operators,
iii) Checking or certifying the calibration of instrumentation,
iv) Harmonising existing test methods,
v) Evaluating new test methods,
vi) Assigning values and ranges to standard materials,
vii) Resolving interlaboratory differences.
1.4. Proficiency testing
When an interlaboratory test comparison is conducted for the express purpose of determining a laboratory’s capability to conduct specific diagnostic tests, i.e. 1.3.i above, it is referred to as proficiency testing. Proficiency testing is an integral part of laboratory accreditation programmes.
Proficiency testing schemes are based on defined sets of highly characterised test materials which are sometimes referred to as check sample panels. These panels are simultaneously sent to participating laboratories for testing. The results are collected and analysed against the intended result in order to determine the capability of a participating laboratory to conduct a diagnostic test and produce correct results.
1.5. Accreditation
An accreditation programme is a formal process for recognition of laboratory quality and capability by an independent authority. It requires that laboratories successfully participate in an accreditation programme on an ongoing basis in order to maintain their recognition status. The independent authority awards or denies recognition based on stipulated requirements for quality and capability.
In the initial stage of accreditation, laboratories are required to demonstrate a specified and sustainable level of quality. Ideally this would involve compliance with ISO 9000 and ISO/IEC 17025 General Requirements for the Competence of Calibration and Testing Laboratories (1990) in order to qualify for entry into the programme. However, it is recognised that in many circumstances such a high level may be difficult to achieve for a variety of reasons. The WOAH Guidelines for Laboratory Quality Evaluation were prepared in order to establish a minimum acceptable level of quality.
The second stage of accreditation entails regularly scheduled proficiency testing for the evaluation of a laboratory’s capability to conduct specific diagnostic tests. As proficiency testing schemes are a form of interlaboratory comparison, they must involve two or more laboratories. There is no agreed standard for proficiency testing in veterinary diagnostics, although several schemes are in operation at international and national levels. The present guidelines have been prepared to be used in conjunction with the WOAH Guidelines for Laboratory Quality Evaluation. Together, these guidelines form an acceptable basis for a quality assurance programme.
2. Authority and recognition
Accreditation programmes and proficiency testing schemes should be operated by an independent authority in order to prevent any bias in the award or denial of recognition.
Participation in an international accreditation programme and proficiency testing scheme should be voluntary. Lack of participation or failure to achieve recognition should not prevent a laboratory from conducting diagnostic tests or a country from entering into trade agreements.
Participation and recognition status should be made available by the independent authority to trading partners only at the request of or with the consent of the participating laboratory or country authority.
Such a programme and scheme may involve a cost to the participating laboratories for this service.
3. Organisation and management
Details of the proficiency testing scheme and its purpose, eligibility of participating laboratories and disposition of the results should be documented by the coordinating organisation to ensure the protection of proprietary rights and confidential information.
A programme manager should have overall responsibility for the operation, quality and security of the proficiency testing scheme.
It is also the responsibility of the programme manager to ensure that laboratories involved in the production of test materials are compliant with the relevant requirements of the ISO 9000 series of standards and ISO/IEC 17025.
Employees should be free from pressure or inducements that might unduly influence the analysis of proficiency testing results or the recognition status of the participating laboratory.
Adequate supervision and security should be provided by staff involved in either the production and distribution of test materials to be used in the proficiency testing scheme or the receipt and analysis of test results submitted by participating laboratories.
4. Standard methods
For the characterisation of test materials to be used in check sample panels, the standard method should meet or exceed the minimum diagnostic performance characteristics required for eligibility as a prescribed test in the WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals.
The standard test should be calibrated against international standard materials, if these are available. Participating laboratories should also be encouraged to calibrate their own assays against the same international standards.
5. Selection and composition of check sample panel
5.1. General principles
For the purpose of selection of test materials for inclusion in the check sample panel, the initial assessment of the status and/or reactivity of the sample will be determined by the producing laboratory, using the standard method.
Acceptance of test materials into the proficiency panel should be based on repeated testing by more than one analyst conducting multiple runs of the test on different days. Sufficient values should be generated to assure the unequivocal status of the test material, including homogeneity.
5.2. Composition of the proficiency panel
The number of test samples that constitute a check sample panel is not well defined. This will be dictated by the type of analysis to be performed on the results and the numbers required to ensure statistical validity.
Irrespective of the type of test, a minimum of three samples should be included:
i) An unequivocal strong positive,
ii) An unequivocal weak positive,
iii) An unequivocal negative.
However, using only three samples of this nature would render the results very predictable after a few rounds of proficiency testing. It would be advisable, therefore, to add at least two more samples to the check sample panel which could be varied from one proficiency test round to the next. This would prevent participating laboratories from anticipating the expected outcome. The additional samples could be different from the above or replicates of the above or a combination.
In planning the overall process for preparation, testing and distribution of test materials and test items, the provider shall provide for, where appropriate, procedures and resources for:
i) material selection;
ii) maintaining suitable environments for preparation and testing of test material;
iii) material preparation;
iv) measuring and testing;
v) calibration/validation of equipment and measurement methods;
vi) assessing test material homogeneity;
vii) assessing test material stability;
viii) organising interlaboratory test comparisons with collaborators, where necessary; (see Note 1 below)
ix) ensuring adequate storage facilities and conditions;
x) ensuring adequate packaging and labelling;
xi) ensuring appropriate transport and distribution arrangements;
xii) statistical analysis of test results and assigning values of measurands and associated uncertainties;
xiii) ensuring adequate reporting service to participants.
6. Statistical analysis
6.1. Types of data
The choice of statistical analysis will in part be determined by the type of data generated by the test method in question. Qualitative data such as ‘positive’, ‘negative’ and/or ‘suspicious’ are somewhat limited in the statistical procedures which may be applied to them. Quantitative data such as end-point titres, and semi-quantitative data such as percentage inhibition values are more flexible with respect to the types of statistical analysis possible.
Irrespective of the type of data to be analysed, it is important that the data from all of the participating laboratories be compatible. In some cases, this may require that participating laboratories be instructed to use a specific dilution series or to express their data against a common standard.
6.2. Assigned values
Either of two approaches may be used:
a) Assigning of target value before issue
In the initial selection of test materials for the check sample panel, the producing laboratory will have assigned a preliminary value, range or status to the sample. For qualitative data, the assigned value may be the only acceptable value. If this is to be the case, then the producing laboratory should verify the status on a battery of tests to increase the confidence that the assigned value is in fact correct. However, as a goal, at least 80% of the participating laboratories should obtain the same result in proficiency tests. For quantitative and semi-quantitative data, the assigned value should be recalculated after proficiency testing results are submitted, and it should be taken as the mean value after removal of outliers.
b) Assigning target value on the consensus value of the returned participants results
6.3. Statistical methods
Many statistical procedures have been applied to interlaboratory comparisons, some being far more sophisticated than others. As a general rule, the statistics being applied should be valid, straightforward and meaningful to the participating laboratories.
Frequency analysis is a simple and meaningful method for participating laboratories to see where their performance lies with respect to the other laboratories in the proficiency testing scheme.
Measures of intra- and interlaboratory variance through repeatability and reproducibility indices will often provide valuable information on the precision and robustness of the test methods.
Youden analysis is a useful indicator of systematic or random error sources that may be causing problems in individual laboratories.
7. Pass/fail criteria
Decision criteria with regards to passing or failing a laboratory on a proficiency test should be clearly documented. These criteria must take into consideration factors which may vary from one disease to another and between types of tests. Once established, the criteria must be applied uniformly.
The types of statistical analyses chosen should assist in making pass/fail decisions. Laboratories submitting results that fall outside ranges established by statistical means should be identified. Results of tests that would potentially lead to a false-negative classification of an infected animal would have to be weighed against results that would potentially lead to a false-positive classification of a healthy animal. In most instances, the former type of error should not be tolerated as it indicates that there is a problem with diagnostic sensitivity. However, there may be some latitude in awarding a provisional status to laboratories experiencing problems with diagnostic specificity.
8. Frequency of proficiency testing
It is recommended that proficiency testing be done on a twice yearly basis, where possible. Depending on the country and disease, some consideration should be given to peak testing periods. Whenever possible, at least one of the proficiency tests should be scheduled to coincide with active testing periods.
Twice yearly, provides sufficient time between proficiency tests to undertake any corrective actions which might prevent a participating laboratory from losing its recognition status.
9. Laboratory recognition
The criteria for awarding, denying or withdrawing recognition should be clearly documented.
10. Logistics
10.1. Eligibility and acceptance
Eligible laboratories should be sent a comprehensive outline of the quality assurance programme and the proficiency testing scheme. This outline should include details pertaining to frequency of testing, commitments and deadlines, methods of data analysis, reporting structure, criteria for recognition, disposition of results and confidentiality. In addition, a form to be signed and returned to the coordinating organisation should be included which indicates that the eligible laboratory accepts the terms and conditions of the programme.
10.2. Notification and shipment of panels
Participating laboratories should be notified at least 1 month in advance of a pending proficiency test. Notification should also include the projected date and method of shipment of the check sample panel. Longer notification may be required by those laboratories in countries requiring import permits for the check sample panels.
Test materials in the check samples should be coded so as not to indicate their expected result. The coding may be alphabetic or numeric. A unique set of codes helps to prevent collusion between laboratories.
All shipments should be by the most expedient and direct method. All shipments should comply with IATA[3] regulations concerning the shipment of biological materials.
Upon shipment, the recipient laboratories should be informed of pertinent details (i.e. method of shipment, carrier, air-way bill, etc.) in order to facilitate rapid retrieval and clearance of the shipment upon arrival.
Check sample panels arriving in a damaged or questionable condition should be replaced immediately.
10.3. Testing and return of results
Participating laboratories should be given an adequate volume of test material and adequate time to complete the testing of the check sample panel to their satisfaction. The panel may be tested more than once and by more than one person in the participating laboratory. However, only one set of results should be returned to the coordinating organisation for analysis. Normally, the person responsible for running the test routinely should be selected to run the check sample panel.
The check sample panel should be accompanied by a complete set of instructions with respect to reconstitution, storage and handling, special testing requirements, data expression and deadline for the submission of results.
Results must be returned in the proper format and on time. Failure to do so could lead to omission from the round of proficiency testing and loss or downgrading of recognition status.
The coordinating organisation should acknowledge receipt of the results and their acceptance into the analysis.
10.4. Analysis and reporting
Analysis and reporting should be completed in a timely fashion after the deadline for the receipt of results.
A general report summarising the results of all of the analyses should be prepared for distribution to all participating laboratories. Participating laboratories should be randomly assigned a code to ensure anonymity in the general report. Individual laboratories should be informed of their unique code for this run of proficiency tests.
Individual laboratories should also receive a summary of their own performance and their recognition status. This summary should indicate clearly all factors contributing to any change in their status. Where the status has been downgraded, it is especially important to indicate real or potential causes which may have contributed to downgrading. In some instances, it may be pertinent to re-issue a second, identical panel after corrective actions have been taken.
A statement of status may also take the form of an official certificate.
All data, results of analyses and the recognition status of participating laboratories should be kept in confidence at all times.
11. Disclosure
The primary purpose of these guidelines is to remove trade barriers and not to create them. It would be expected that participating laboratories having achieved full recognition status may request that official verification of their status be made available to trading partners from the independent authority or coordinating organisation. This should only be done at the request of or with the consent of the participating laboratory or country authority.
12. References
ISO/IEC International Standard 17025 (2005). General requirements for the competence of testing and calibration laboratories. International Organisation for Standardisation (ISO)/International Electrotechnical Commission (ISO/IEC), ISO Central Secretariat, 1 rue de Varembé, Case Postale 56, CH – 1211, Geneva 20, Switzerland.
International Organisation for Standardisation (ISO) (1997). Proficiency testing by interlaboratory comparisons. Part 1: Development and operation of proficiency testing schemes. Part 2: Selection and use of proficiency testing schemes by laboratory accreditation bodies. ISO/International Electrotechnical Commission (ISO/IEC), Guide 43. ISO/IEC, Geneva, 19 pp.
ISO International Standards 9000:2005, 9001:2000; 9004:2000 (2000–2005). Quality management and quality assurance. International Organization for Standardization (ISO), ISO Central Secretariat, 1 rue de Varembé, Case Postale 56, CH – 1211, Geneva 20, Switzerland
[1] ISO: International Organisation for Standardisation
[2] IEC: International Electrotechnical Commission
[3] IATA: International Air Transport Association
Criteria
The criteria to be applied in the selection of institutions for designation as an WOAH Reference Centre are as follows:
- the institution’s ability, capacity and readiness to provide those services described under the Terms of Reference for WOAH Reference Centres that are intended to form the basis of their relationship with the Organisation, including, for example, the ability to receive biological samples from other WOAH Member Countries;
- the scientific and technical standing of the institution concerned at the national and international levels, presence of veterinary experts within scientific teams and, for Reference Laboratories, conformity with WOAH and other international standards for laboratory quality assurance, biosafety and biosecurity measures;
- the place the institution occupies in the Member’s animal health, scientific or educational structures;
- the quality of its scientific and technical leadership including internationally recognised expertise in the field of its competence, and, for Collaborating Centres, the number and qualifications of its staff;
- the institution’s prospective stability in terms of personnel, activity and funding;
- the working relationship which the institution has developed with other institutions in the territory of the Member, as well as at the regional and global levels;
- the technical and geographical relevance of the institution and its activities to WOAH’s programme priorities.
Internal rules
ARTICLE 1
Applications for the title of Reference Centre of the World Organisation for Animal Health (WOAH) shall be submitted to the Director General by the Delegate of the WOAH Member Country to which the institution belongs or by the corresponding Regional Commission.
ARTICLE 2
The head of the institution shall provide the Director General with a statement of interest for the institution and its staff covering potential conflicts of interest between it as an WOAH institution and any commercial entity in accordance with the procedure established by the Director General. The head of the institution shall ensure that the institution and its staff respect the legitimate confidentiality of information with which they may be entrusted in the performance of their functions for the WOAH and shall submit such an undertaking to the Director General.
A Reference Laboratory should respect the intellectual property rights on samples received and not use those results, without consent, for more than determining the principal characteristics of the pathogen necessary for the country of origin to carry out an epidemiological inquiry and to decide about its control strategy. In the case of positive results for diseases that are reportable to WOAH, the Reference Laboratory should immediately inform the Delegate of the WOAH Member Country from which the samples originated, as well as the WOAH Headquarters.
ARTICLE 3
Applications received shall be presented by the Director General to the Council for endorsement, after consultation with the relevant Regional and Specialist Commissions. Applications shall be selected on the basis of the criteria given above. However, in principle, no more than one Reference Laboratory shall be designated for the same pathogen or disease in the same country and no more than one Collaborating Centre shall be designated for the same category of specialty in the same region or, exceptionally, in a sub-region.
ARTICLE 4
Applications endorsed by the Council shall be presented to the Assembly for approval.
ARTICLE 5
The Director General shall notify approved institutions of their designation as an WOAH Reference Centre, with a formal title to be used as an WOAH Reference Centre. The Director General shall also inform the WOAH Delegate of the host Member Country accordingly.
ARTICLE 6
This notification shall confer on the institution the right to use the title ‘WOAH Reference Laboratory or ‘WOAH Collaborating Centre’ as appropriate and the WOAH emblem on all documents issued by the Reference Centre in its official capacity, and for the Reference Laboratory, the right of the designated specialist to use the title of WOAH Expert.
ARTICLE 7
The Head of the Reference Centre shall be responsible for the overall implementation of the terms of reference, and for a Collaborating Centre, shall act as the sole interface with the WOAH. For a Reference Laboratory, the WOAH Expert is responsible for the implementation of the technical aspects of the terms of reference and may delegate specific responsibilities to other experts on an ad hoc basis. Experts associated with WOAH Reference Centres exercise their function within the rules applicable to WOAH Experts.
ARTICLE 8
The Reference Centre shall provide to the Director General a brief report of activities related to their terms of reference at the end of each calendar year, according to the template established by the WOAH Headquarters. This report will be made available to all Member Countries.
ARTICLE 9
The Reference Centre may revoke the designation at any time. The designation shall be withdrawn if the Reference Centre fails to comply with the provisions of the Terms of Reference and the present Rules. In such cases, the Director General of the WOAH, after consulting an appropriate Specialist Commission, proposes the withdrawal to the World Assembly of Delegates.
ARTICLE 10
Any major change within the institution which may impair the function of the Reference Centre (particularly changes in personnel and in material or financial resources) shall be reported immediately to the Director General who will consult the relevant Regional and Specialist Commissions on the continuing status of the institution as a Reference Centre.
WOAH Reference Laboratories must provide evidence of scientific leadership and of the capability to fulfil the Terms of Reference: all applicants should preferably be the national reference laboratory; they should be able to receive samples from other countries for diagnostic testing; they should demonstrate the capability and willingness to organise rather than just participate in proficiency tests; they should be capable of providing confirmatory diagnostic services, reference materials, training, etc., internationally; and the designated expert should have a number of recent relevant publications in peer-reviewed journals. A functioning Reference Laboratory requires input from experts in a number of different fields working on the same disease or pathogen. It is understood that the WOAH designated expert is a leading member of such a multidisciplinary team who could consult other members of this team with different expertise in response to requests received while remaining the single contact point for all correspondence with WOAH Members and others. Article 7 of the Internal Rules for WOAH Reference Centres captures this idea when it states “For a Reference Laboratory, the WOAH Expert is responsible for the implementation of the technical aspects of the terms of reference and may delegate specific responsibilities to other experts on an ad hoc basis.”
Applications should be submitted 45 days before the date scheduled for the meetings of the relevant Specialist Commission: both the Biological Standards Commission and the Aquatic Animal Health Standards Commission meet in February and September; the deadlines are therefore mid-December and mid-July. The 45-day period gives the WOAH sufficient time to screen, translate into English when necessary, and process the dossiers for the Commission’s evaluation. Deadlines must be strictly observed to allow a full evaluation of the dossiers by the members of the Commission prior to its meeting. Applications received after the deadline will be examined in the next meeting of the Commission.
Applications shall be submitted in accordance with Article 1 of the Internal Rules and should include the following information:
Administration and management
1. Name of expert (a curriculum vitae using this template).
2. Name and address of laboratory (telephone and e-mail address [fax numbers or Web site, if available]).
3. Name of the Head of laboratory (Responsible Official).
4. Demonstrate that legal and budgetary provisions are in place that provide assurance on the sustainability and functioning of the laboratory.
5. Provide documented proof (certificates) of accreditation to the ISO 17025 or equivalent quality management system, ideally with relevant tests included in the scope of the accreditation.
Technical expertise and experience
6. Give an overview of the expertise available in the disease- or pathogen-related multidisciplinary team in which the expert works: disciplines, number of experts in each discipline, level of expertise.
7. Give details of experience in diagnostic testing for the disease according to the WOAH Standards nationally and internationally (approximate number of tests performed annually for each technique).
8. Provide additional information on expertise in diagnostic techniques (agent characterisation techniques, molecular techniques, monoclonal antibody techniques, etc.), epidemiology and control of the disease.
9. Give details of experience in standardisation and validation of diagnostic tests.
10. Demonstrate reagent production capability (provide details of current stock of reagents for the disease).
11. Demonstrate capability for timely international shipment and receipt of samples in accordance with the requirements for postage and packaging of biological materials described in the WOAH Manual of Diagnostic Tests and vaccines for Terrestrial Animals, and the WOAH Terrestrial Animal Health Code or the WOAH Aquatic Animal Health Code.
12. Provide a list of completed research and methods development projects on the disease.
13. Provide a list of inter-laboratory proficiency tests that the laboratory regularly organises and participates in.
14. Give details of training and consultation experience for the disease in the last 2 years (courses provided, number of people trained, examples of international consultation).
15. Provide a list of scientific meetings that the laboratory has organised and participated in.
16. Provide a list of reference documents (chapters for the WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, WOAH Manual of Diagnostic Tests for Aquatic Animals, etc.) to which the laboratory contributed.
Collaborations, confidentiality and conflicts
17. Provide a list of collaboration agreements with other laboratories, centres or organisations.
18. Provide guarantees to ensure that staff respect the confidential nature of certain subjects, results or communications, and with regard to management of potential conflict of interests through completion of the required declarations.
19. Provide a Confidentiality Undertaking and a Declaration of Interests form signed by the Head of the Institution on behalf of the Institution, in accordance with the Rules for WOAH Reference Laboratories.
The application will be processed by WOAH in accordance with Articles 2, 3 and 4 of the Internal Rules.
A short summary of activities of relevance to the status of WOAH Reference Laboratory (no more than one page) should be included.
Applications comprising the information requested in all the above-mentioned points must be no longer than 15–20 pages in A4 format, single-spaced using Times New Roman font size 10 pt. The application must be prepared in one of the official languages of the WOAH (English, French or Spanish).
The WOAH, as a data controller, will process your contact and identification details obtained from privately held sources for pursuing our mandate, including for communications, meeting management, and training. Furthermore, when publishing the lists of designated WOAH Reference Centres and annual reports of their activities, the WOAH will include your personal details on the WOAH website. The data collected will be processed internally for the aforementioned purposes. Moreover, as an international organisation, we transfer personal data within our various offices as well as to third party service providers and partners to perform tasks on our behalf and to assist us in pursuing our mandate. Your personal data shall be kept until no longer relevant. You have rights to access and rectify your personal data, as well as to request erasure, object to processing and obtain data portability under certain circumstances. You can find more information regarding the processing and your rights on our Privacy Policy.
In the context of the performance of your obligations as an WOAH expert, you may occasionally be required to process personal data, on behalf of the WOAH.
Should you be required to process personal data on behalf of the WOAH, you must provide sufficient guarantees as to the implementation of appropriate technical and organisational measures, so that the processing operations comply with generally accepted standards on data protection.
When processing personal data on behalf of the WOAH, you declare that you provide sufficient guarantees as to the implementation of the appropriate technical and organisational measures, so that the processing operations fully comply with the requirements of the applicable regulations on the protection of personal data.
It is expressly agreed that you:
- process the data only for the purpose(s) covered by your mandate and the instructions concerning the processing sent by the WOAH;
- may only process the personal data on documented instruction from the WOAH, including with regard to the location of the hosting and transfers to third countries;
- guarantee the confidentiality of the personal data processed under instruction from the WOAH;
- help the WOAH, through appropriate technical and organisational measures, insofar as this is possible, to fulfil its obligation to comply with any requests of relevant persons concerning their rights (rights of access, rectification, erasure and objection, restriction of processing, data portability) and to fulfil its obligation to notify personal data breaches when it deems it fit;
- take and implement all appropriate measures to ensure a level of security appropriate to the risk presented by the processing operations;
- on request of the WOAH, delete all personal data or return them to the WOAH or to the new expert after the end of your mandate; the return of such data must be accompanied by the destruction by you of all existing copies in the latter’s information systems.
Guidance for the Management of WOAH Reference Laboratory Networks
Introduction
The global network of WOAH Reference Centres is the central core of the WOAH’s scientific excellence. The First International Conference of WOAH Reference Laboratories and Collaborating Centres (held in Florianopolis, Brazil, in 2006) recommended that a network of WOAH Reference Laboratories and Collaborating Centres be developed with the objective of harmonising and exchanging data, information and reference material to improve disease surveillance and control worldwide.
The Second Global Conference of WOAH Reference Laboratories and Collaborating Centres (held in Paris in June 2010) encouraged the networks of WOAH Reference Laboratories and Collaborating Centres to continue working together to strengthen multilateral cooperation, in particular with the aim of producing and increasing availability of validated biological reference materials. In order to strengthen this collaboration, the WOAH Reference Laboratories and Collaborating Centres should continue to exchange knowledge, reference materials and expertise to the benefit of WOAH Member Countries.
The Terms of Reference (ToR) adopted in May 2011 explicitly require WOAH Reference Laboratories to establish and maintain a network among all the WOAH Reference Laboratories designated for the same pathogen. More details on the ToR of WOAH Reference Laboratories are available at the following link.
The WOAH has identified the need for further guidance on the coordination of the WOAH Reference Centre networks. The objective is to assure unified expert opinions and advice to WOAH Member Countries through the enhanced exchange of information.
The network of WOAH Reference Laboratories shall assure consistent results across laboratories through the adherence to WOAH Standards, the sharing of reference materials within the network and the participation in appropriate proficiency testing. Networking also provides opportunities for exchange of knowledge and experience, mutual support and development of scientific collaborations. It will improve the credibility and increase the visibility of WOAH Reference Laboratories worldwide, and will attract the participation of other national reference laboratories from WOAH Member Countries.
On a case-by-case basis, the WOAH Headquarters could invite the existing Reference Laboratories to meet (physically or by telephone conference) to help to create a network, to appoint the secretariat, and to follow this guidance.
Recommendations:
- Networking among WOAH Reference Laboratories is part of their ToR. When two or more WOAH Reference Laboratories are designated for the same pathogen, a network must be established. Participation in the network is compulsory for WOAH Reference Laboratories. Only WOAH Reference Laboratories are accountable to the WOAH. However other reference laboratories may participate in some of the activities of the network, as appropriate.
- The network should have a secretariat (officially notified to the WOAH) in one of the participating WOAH Reference Laboratories to serve as a liaison with the WOAH Headquarters. It is recommended that the secretariat is responsible for coordination, leadership and accountability of the network. The secretariat may rotate among participating laboratories (e.g. every 3 years). It is the responsibility of the Secretariat of the network to manage conflict of interests and confidentiality declarations if they are deemed required by the network and WOAH is not involved in this process.
- Each network should have a clear work plan and the secretariat should provide the WOAH Director General with an annual report of its activities : achievements, obstacles, future initiatives (individual laboratories can make reference to the network report in its annual WOAH Reference Laboratory report).
- When meetings are organised by the network, participation of WOAH staff as observers should be allowed, and the secretariat should produce a meeting report that should be shared with the WOAH Headquarters.
- If a discrepancy or disagreement arises that cannot be resolved within the network, the secretariat should inform the WOAH Headquarters without delay. The WOAH Headquarters will inform the Biological Standards Commission accordingly.
- Conditions for inclusion of a network website link on the Website of the WOAH:
- The network may establish a website to disseminate information on its activities. The network should formally request the Director General of the WOAH that a link to its website be added to the WOAH website.
- The website of the network must comply with WOAH rules for graphic layout and other applicable WOAH policies.
- Any major changes to the website of the network should be notified to the WOAH in advance.
- The WOAH Headquarters reserves the right to recommend any changes to the content of the web site of the network, as deemed appropriate, and to remove the link of the web site of the network at any time while providing the reasons to the secretariat of the network.
- The reports and activities of the network reflects the views of the network members and may not necessarily reflect the views of the WOAH.
WOAH contact point for networks:
Sara Linnane
Science Department
World Organisation for Animal Health (WOAH)
12, rue de Prony
75017 Paris, France
E-mail: [email protected]