Terrestrial Animal Health Code
Terrestrial Animal Health Code |
Import risk analysis
Introduction
The importation of animals and animal products involves a certain level of disease risk to the importing country. This risk may be represented by one or several diseases, infections or infestations.
The principal aim of import risk analysis is to provide importing countries with an objective and defensible method of assessing the disease risks associated with the importation of animals, animal products, animal genetic material, feedstuffs, biological products and pathological material. The analysis should be transparent. Transparency means the comprehensive documentation and communication of all data, information, assumptions, methods, results, discussion and conclusions used in the risk analysis. This is necessary so that the exporting country and all interested parties are provided with clear reasons for the imposition of import conditions or refusal to import.
Transparency is also essential because data are often uncertain or incomplete and, without full documentation, the distinction between facts and the analyst's value judgements may blur.
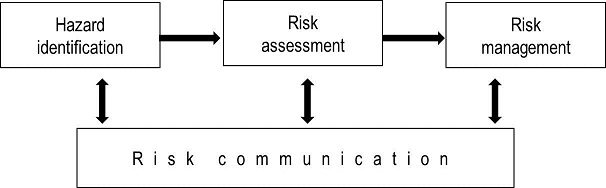
This chapter provides recommendations and principles for conducting transparent, objective and defensible risk analyses for international trade. The components of risk analysis are hazard identification, risk assessment, risk management and risk communication (Figure 1).
Fig. 1. The four components of risk analysis
The risk assessment is the component of the analysis which estimates the risks associated with a hazard. Risk assessments may be qualitative or quantitative. For many diseases, particularly for those diseases listed in this Terrestrial Code where there are well developed internationally agreed standards, there is broad agreement concerning the likely risks. In such cases it is more likely that a qualitative assessment is all that is required. Qualitative assessment does not require mathematical modelling skills to carry out and so is often the type of assessment used for routine decision making. No single method of import risk assessment has proven applicable in all situations, and different methods may be appropriate in different circumstances.
The process of import risk analysis usually needs to take into consideration the results of an evaluation of Veterinary Services, zoning, compartmentalisation and surveillance systems in place for monitoring of animal health in the exporting country. These are described in separate chapters in the Terrestrial Code.
Hazard identification
The hazard identification involves identifying the pathogenic agents which could potentially produce adverse consequences associated with the importation of a commodity.
The hazards identified would be those appropriate to the species being imported, or from which the commodity is derived, and which may be present in the exporting country. It is then necessary to identify whether each hazard is already present in the importing country, and whether it is a notifiable disease or is subject to control or eradication in that country and to ensure that import measures are not more trade restrictive than those applied within the country.
Hazard identification is a categorisation step, identifying biological agents dichotomously as hazards or not. The risk assessment may be concluded if hazard identification fails to identify hazards associated with the importation.
The evaluation of the Veterinary Services, surveillance and control programmes and zoning and compartmentalisation systems are important inputs for assessing the likelihood of hazards being present in the animal population of the exporting country.
An importing country may decide to permit the importation using the appropriate sanitary standards recommended in the Terrestrial Code, thus eliminating the need for a risk assessment.
Principles of risk assessment
Risk assessment should be flexible to deal with the complexity of real life situations. No single method is applicable in all cases. Risk assessment should be able to accommodate the variety of animal commodities, the multiple hazards that may be identified with an importation and the specificity of each disease, detection and surveillance systems, exposure scenarios and types and amounts of data and information.
Both qualitative risk assessment and quantitative risk assessment methods are valid.
The risk assessment should
be based on the best available information that is in accord with
current scientific thinking. The assessment should be well-documented
and supported with references to the scientific literature and other
sources, including expert opinion.
Consistency in risk assessment methods should be encouraged and transparency is essential in order to ensure fairness and rationality, consistency in decision making and ease of understanding by all the interested parties.
Risk assessments should document the uncertainties, the assumptions made, and the effect of these on the final risk estimate.
Risk increases with increasing volume of commodity imported.
The risk assessment should be amenable to updating when additional information becomes available.
Risk assessment steps
Entry assessment
Entry assessment consists of describing the biological pathways necessary for an importation activity to introduce pathogenic agents into a particular environment, and estimating the probability of that complete process occurring, either qualitatively (in words) or quantitatively (as a numerical estimate). The entry assessment describes the probability of the ‘entry’ of each of the hazards (the pathogenic agents) under each specified set of conditions with respect to amounts and timing, and how these might change as a result of various actions, events or measures. Examples of the kind of inputs that may be required in the entry assessment are:
Biological factors
species, age and breed of animals
agent predilection sites
vaccination, testing, treatment and quarantine.
Country factors
incidence or prevalence
evaluation of Veterinary Services, surveillance and control programmes and zoning and compartmentalisation systems of the exporting country.
Commodity factors
quantity of commodity to be imported
ease of contamination
effect of processing
effect of storage and transport.
If the entry assessment demonstrates no significant risk, the risk assessment does not need to continue.
Exposure assessment
Exposure assessment consists of describing the biological pathways necessary for exposure of animals and humans in the importing country to the hazards (in this case the pathogenic agents) from a given risk source, and estimating the probability of the exposures occurring, either qualitatively (in words) or quantitatively (as a numerical estimate).
The probability of exposure to the identified hazards is estimated for specified exposure conditions with respect to amounts, timing, frequency, duration of exposure, routes of exposure, such as ingestion, inhalation or insect bite, and the number, species and other characteristics of the animal and human populations exposed. Examples of the kind of inputs that may be required in the exposure assessment are:
Biological factors
properties of the agent.
Country factors
presence of potential vectors
human and animal demographics
customs and cultural practices
geographical and environmental characteristics.
Commodity factors
If the exposure assessment demonstrates no significant risk, the risk assessment may conclude at this step.
Consequence assessment
Consequence assessment consists of describing the relationship between specified exposures to a biological agent and the consequences of those exposures. A causal process should exist by which exposures produce adverse health or environmental consequences, which may in turn lead to socio-economic consequences. The consequence assessment describes the potential consequences of a given exposure and estimates the probability of them occurring. This estimate may be either qualitative (in words) or quantitative (a numerical estimate). Examples of consequences include:
Direct consequences
animal infection, disease and production losses
public health consequences.
Indirect consequences
surveillance and control costs
compensation costs
potential trade losses
adverse consequences to the environment.
Risk estimation
Risk estimation consists of integrating the results from the entry assessment, exposure assessment, and consequence assessment to produce overall measures of risks associated with the hazards identified at the outset. Thus risk estimation takes into account the whole of the risk pathway from hazard identified to unwanted outcome.
For a quantitative assessment, the final outputs may include:
estimated numbers of herds, flocks, animals or people likely to experience health impacts of various degrees of severity over time;
probability distributions, confidence intervals, and other means for expressing the uncertainties in these estimates;
portrayal of the variance of all model inputs;
a sensitivity analysis to rank the inputs as to their contribution to the variance of the risk estimation output;
analysis of the dependence and correlation between model inputs.
Principles of risk management
Risk management is the process of deciding upon and implementing measures to address the risks identified in the risk assessment, whilst at the same time ensuring that negative effects on trade are minimised. The objective is to manage risk appropriately to ensure that a balance is achieved between a country's desire to minimise the likelihood or frequency of disease incursions and their consequences and its desire to import commodities and fulfil its obligations under international trade agreements.
The international standards of the OIE are the preferred choice of sanitary measures for risk management. The application of these sanitary measures should be in accordance with the intentions in the standards.
Risk management components
Risk evaluation - the process of comparing the risk estimated in the risk assessment with the reduction in risk expected from the proposed risk management measures.
Option evaluation - the process of identifying, evaluating the efficacy and feasibility of, and selecting measures to reduce the risk associated with an importation. The efficacy is the degree to which an option reduces the likelihood or magnitude of adverse health and economic consequences. Evaluating the efficacy of the options selected is an iterative process that involves their incorporation into the risk assessment and then comparing the resulting level of risk with that considered acceptable. The evaluation for feasibility normally focuses on technical, operational and economic factors affecting the implementation of the risk management options.
Implementation - the process of following through with the risk management decision and ensuring that the risk management measures are in place.
Monitoring and review - the ongoing process by which the risk management measures are continuously audited to ensure that they are achieving the results intended.
Principles of risk communication
Risk communication is the process by which information and opinions regarding hazards and risks are gathered from potentially affected and interested parties during a risk analysis, and by which the results of the risk assessment and proposed risk management measures are communicated to the decision-makers and interested parties in the importing and exporting countries. It is a multidimensional and iterative process and should ideally begin at the start of the risk analysis process and continue throughout.
A risk communication strategy should be put in place at the start of each risk analysis.
The communication of the risk should be an open, interactive, iterative and transparent exchange of information that may continue after the decision on importation.
The principal participants in risk communication include the authorities in the exporting country and other stakeholders such as domestic and foreign industry groups, domestic livestock producers and consumer groups.
The assumptions and uncertainty in the model, model inputs and the risk estimates of the risk assessment should be communicated.
Peer review is a component of risk communication in order to obtain scientific critique and to ensure that the data, information, methods and assumptions are the best available.
nb: first adopted in 1998; most recent update adopted in 2018.
2018 ©OIE - Terrestrial Animal Health Code |