Antimicrobial resistance
Imagine a world where infections and diseases in humans, animals and plants are impossible to treat. This worst-case scenario could become a reality as bacteria, viruses and parasites develop resistance to the drugs we use to fight them. Antimicrobial resistance, or AMR, has become one of the most pressing health issues of our time. Solutions exist and everyone has a role to play in the fight against this global threat.
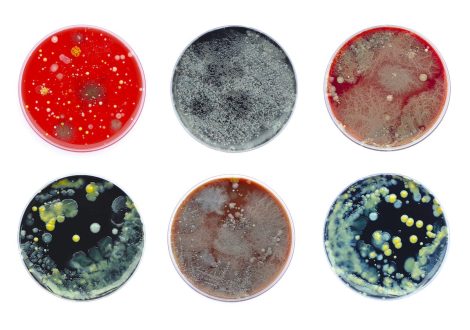
Antimicrobial resistance is a growing threat to animal and human well-being
They rank among humanity’s most spectacular achievements. Antimicrobial drugs, such as antibiotics that are used to treat bacterial infections, paved the way to better living conditions for humans and animals. Before modern medicine, infections due to minor cuts could lead to bloodstream infections or death.
Today, antimicrobials help animals and humans live longer and healthier lives. But how long will this last? Many of these life-saving drugs are losing their efficacy as previously susceptible microbes (bacteria, virus, fungi, and microscopic parasites) become resistant. The phenomenon is known as “antimicrobial resistance” or AMR. Antimicrobial resistance has led to the emergence of so-called “superbugs”, that are challenging health care workers, veterinarians, and other animal health providers due to a reduction of effective therapeutic options to prevent, control, and treat infectious diseases. Animals and humans are becoming helpless, once again, in the face of infection.
By reversing decades of progress, antimicrobial resistance is one of the greatest global health challenges of our time, becoming a leading cause of death globally. It is a growing threat to animal and human health, as well as livelihoods and food security worldwide.
The good news is that solutions exist to curb the emergence of drug-resistant microbes. And everyone–farmers, aquatic animal producers, animal health providers, health care workers, citizens from every country can take action to fight this threat to global health.
What are the impacts of AMR on animal, human, plant, and environmental health?
The spread of new resistant strains of bacteria in terrestrial and aquatic animals leads inexorably to an increase in animal suffering and losses. This in turn affects livelihoods worldwide, as 1.3 billion people rely on livestock for their living and over 20 million people depend on aquaculture.
When antibiotics spill into the soil and waterways, resistant strains of bacteria can emerge in the environment. They in turn can infect animals and humans that come into contact with them. Also, antibiotic resistant bacteria of treated animals can be present in manure and, therefore, be disseminated into the environment and to wildlife. Responsible use of antibiotics and proper disposal of unused and expired drugs, as well as waste from relevant industries ensures that these precious pharmaceuticals stay out of the environment as much as possible and reduces the risk of development of resistant bacteria.
The same phenomenon can be observed in human health as well, with antimicrobial resistance emerging from misuse of antibiotics in people. Today, new resistant strains of bacteria dangerously affect hospital patients all over the world. Infections such as gonorrhoeae, cystitis or infections linked to routine surgeries such as hip replacement, are becoming harder to cure in humans. It still remains unclear how many human deaths are linked to AMR originated in animals, notably through food-borne infections.
In order to ensure the efficacy of antimicrobials and secure the health and development gains of these last 50 years, antimicrobial resistance must be contained.
An estimated
5 million
human deaths were linked to antimicrobial resistance in 2019
Including
1.3 million
human deaths directly caused by resistant bacteria
Murray CJL et al. The Lancet 2022, Vol. 399, Issue 10325. doi:10.1016/S0140-6736(21)02724-0.
While the current burden of disease in animals due to AMR at global level remains unknown, several initiatives are ongoing to estimate it, notably the Global Burden of Animal Diseases programme in which we are actively involved.
How do bacteria become resistant to drugs?
It is a race between humans, who try to cure sickness, and germs such as bacteria, that evolve to survive. Antibiotics work by killing or limiting the growth of the bacteria that make humans and animals sick. They cure animal diseases such as mastitis in dairy cows, respiratory and urinary tract infections in dogs or streptococcal infections in fish and are key to reducing animal suffering and death. Yet, bacteria are very good at adapting to their environments over time. By random genetic mutations and transfer of antimicrobial resistance traits, they can sometimes acquire genes that enable them to survive drugs intended to kill them. Through natural selection, new resistant variants can thrive and spread. Every time antibiotics are used, bacteria get a chance to develop resistance. Does this mean we should stop using antibiotics? Absolutely not, but it does mean that we need to use them responsibly, and only when necessary.
Antibiotics are vital to global health, and to stop using them when medically justified, is not an option. It is our duty to preserve animal health and welfare. However, in too many cases, antibiotics are misused, needlessly creating the conditions in which drug-resistance can emerge. Using an antibiotic to treat a cow’s viral infection, for instance, will be of no use to the cow as antibiotics are effective against bacteria but not viruses. Antibiotics are also sometimes overused to promote growth in food producing animals. Misuse and overuse could lead antibiotics to cause more harm than good. But by using these drugs responsibly–and only when necessary–we can reduce the pathogens’ chances of developing resistance, and protect the health of humans, animals, plants, and the environment.
The solution: prevention and responsible use of antimicrobials
Curbing antimicrobial resistance may seem daunting. However, we already know how to make it happen. In the animal health sector, several measures can be implemented by farmers, aquatic animal producers, pet owners and relevant professionals to ensure that these precious drugs are used responsibly and remain effective in the future.
Animals are more susceptible to diseases when they live in stressful environments or when the hygiene conditions are poor. Therefore, following good animal management practices focusing on disease prevention, and using antimicrobials responsibly is essential. Doing so, we can collectively limit the development of antimicrobial resistance and protect the efficacy of antimicrobials for future generations of animals and humans. A growing number of farmers and animal health professionals worldwide are already changing their practices to successfully address the threat of AMR. These efforts are inspiring and protecting everyone. Let’s follow these examples as much more still needs to be done.
One Health: we all have a role to play in curbing the rise of AMR
Animal health, human health, and environmental health are intrinsically intertwined and interdependent. We share the land, resources… and pathogens. Dangerous strains of resistant bacteria can spread between and within animal, human and plant populations and travel through the waterways, soil, and air, infecting wild animals along the way. As more than 60% of pathogens that cause human diseases originate from domestic animals or wildlife, protecting the health of animals and the environment protects human health.
Fighting antimicrobial resistance is a truly global endeavour and must be addressed through a One Health approach. This is why collaboration between sectors dealing with human, animal, plant and environmental health is crucial.
It is by reducing the overuse of antimicrobials in humans, animals, and plants that we will be able to achieve better global health.
Questions and answers about antimicrobial resistance
Microbes are very small organisms that cannot be seen at naked eye and can only be observed through a microscope. They include bacteria, viruses, fungi and microparasites.
A pathogen is an organism that can make humans, animals, and plants sick. They can be bacteria, viruses, fungi and parasites. However, many of them are harmless and, therefore, not considered pathogens. For instance, many of the non-pathogenic bacteria are part of the normal gut flora of animals and humans and are known as commensal bacteria. These can contribute to the normal functioning of the gut.
Antimicrobial resistance is a phenomenon driven by random mutations and natural selection. Some bacteria are also able to share genetic material with other bacteria, increasing the spread of resistance across bacterial populations in humans, animals, plants, and through the environment. Antimicrobial resistance is greatly accelerated by the improper use of antimicrobials, as these can exert selective pressure for bacteria with resistance traits to survive and thrive.
Everyone is affected by the rise of antimicrobial resistance worldwide. It is a global problem that threatens the health of humans, animals, plants, and the environment. The impact of AMR is higher in low- and middle-income countries with reduced access to healthcare or veterinary services and where the use of antimicrobials is poorly regulated and controlled due to limited resources.
No. Antibiotics, when used properly, treat bacterial but not viral infections.
While all antibiotics are antimicrobials, not all antimicrobials are antibiotics. An antimicrobial is a substance that kills pathogens or stops their growth. Antibiotics are a specific type of antimicrobial that are used against bacteria. In the same way, antifungals are used against fungi. Both antibiotics and antifungals are antimicrobials.
No. We are responsible for the well-being of our domestic animals and our health is linked to their health. Prohibiting the use of antimicrobials in animals would seriously compromise animal health and welfare, food security as well as the livelihoods of farming communities. This would consequently have a negative impact on national economies and food security. By using antimicrobials responsibly in animals and humans, we can preserve their efficacy for all.
No. Nothing can stop bacteria or other pathogens from adapting to their environment and developing resistance. However, it is possible to slow the process enough for it to be a manageable problem and to protect the efficacy of antimicrobials needed to treat infections in animals, humans, and plants.
No. Antimicrobials cure sick animals and humans and must be used according to existing guidelines. It is by reducing overuse and misuse that we can curb antimicrobial resistance. In fact, we can prevent disease in animals and humans through vaccination programmes or through the implementation of other relevant measures, such as biosecurity measures in farms to prevent the introduction of infectious diseases in animal populations.
You produce, use, or prescribe antimicrobials and want to improve your practices:
- Follow our guidelines for responsible use of antimicrobials
- Spread the word about antimicrobial resistance and its solutions
You are a citizen interested in helping.
Here’s what you can do:
- Use antimicrobials (such as antibiotics) as prescribed by a vet
- Learn about antimicrobial resistance
- Spread the word
Leading the fight against antimicrobial resistance
A global threat calls for a global, coordinated response. The World Organisation for Animal Health (WOAH) is uniquely positioned to help lead the global fight against antimicrobial resistance. Whether it is by closely working with national Veterinary and Aquatic Animal Health Services, collecting and analysing data on antimicrobial use in animals, or advocating for improved practices, we are acting to steer the world towards a healthier and more sustainable future.
Our AMR strategy presented by the Director General
Four pillars to curb the spread of AMR
Support good governance and capacity building
Encourage implementation of International Standards
Strengthen knowledge through surveillance and research
Improve AMR awareness and understanding
Enhancing Veterinary Services’ capacity to address AMR
Veterinarians and aquatic animal health professionals are the first line of defense when it comes to curbing the spread of antimicrobial resistance in the animal health sector. They care for sick animals, both terrestrial and aquatic, and decide when and how to administer antibiotics and other antimicrobials.
They also provide advice on how to raise animals following good animal husbandry and biosecurity practices to prevent and control diseases so as to rationalise the need for antibiotics. Our international Standards provide guidance to national Animal Health Services to support the effective treatment of animals while limiting the emergence of drug-resistant strains of pathogens.
Animal Health Services worldwide can acquire these good practices through specific AMR capacity building sessions and workshops. They can also access reference documents such as the list of antimicrobials of veterinary importance, which provides specific recommendations on how to use in veterinary medicine certain pharmaceuticals considered as high priority for prevention and control of infections in humans.
Only effective and competent national Animal Health Services can meet the challenges of animal health and welfare whilst protecting everyone from the threat of drug-resistant pathogens. Therefore, the World Organisation for Animal Health strives to help countries improve the overall efficacy of their national animal health systems.
Our flagship programme, the Performance of Veterinary Services Pathway (PVS Pathway), provides national Animal Health Services with a comprehensive understanding of their strengths and weaknesses in all the areas of work which are under their responsibility, including antimicrobial resistance. It then supports them in best addressing the gaps identified and prioritising their actions.
Once countries have identified what is needed to fight AMR more effectively, they can sometimes be hindered by a lack of financial resources. The AMR Multi-Partner Trust Fund, managed jointly with our partners, supports efforts to battle AMR in low- and middle-income countries following a multisectoral, One Health approach in the implementation of the National Action Plans on AMR. The financed projects help raise awareness among relevant audiences and support and strengthen the development and implementation of monitoring and surveillance programmes for antimicrobial use and AMR within and across sectors, among other actions.
WOAH also develops advocacy initiatives to support Veterinary Services in their dialogue with national authorities. In this line, we have published a position statement urging the animal health sector to actively initiate the phasing out of the use of antimicrobials as growth promoters in animals.
Establishing International Standards for a responsible use of antimicrobials
Our International Standards are crafted as guidelines to improve animal health and welfare. Following them reduces the burden of infectious diseases in animal populations, therefore reducing the need and use of antimicrobials. And by using fewer antimicrobials, we limit the conditions in which bacteria and other pathogens can develop resistance. However, these Standards need to be successfully implemented in the field to fulfil their purpose.
One of the most effective approaches to guarantee their implementation is through legislation. Our International Standards should serve as a basis for national regulations. As an essential element of a nation’s infrastructure, veterinary legislation provides the powers necessary for Veterinary Authorities to ensure animal and public health. The implementation of appropriate legislation can, for instance, ensure that countries take a strong stance against the use of falsified medicines or ban over-the counter sales of specific pharmaceuticals.
Yet, gaps exist in national AMR legislations that might impinge on the Veterinary Services ability to carry out their mission. Our Veterinary Legislation Support Programme (VLSP) helps Members recognise and address their needs for clear, comprehensive veterinary legislation. Because a strong legal framework is necessary if countries are to take effective action in the face of health threats such as AMR.
WOAH’s International Standards for controlling antimicrobial resistance
Terrestrial Animal Health Code
Chapter 6.7 Introduction to the recommendations for controlling antimicrobial resistance
Chapter 6.8 Harmonisation of national antimicrobial resistance surveillance and monitoring programmes
Chapter 6.9 Monitoring of the quantities and usage patterns of antimicrobials agents used in food producing animals
Chapter 6.10 Responsible and prudent use of antimicrobial agents in veterinary medicine
Chapter 6.11 Risk analysis for antimicrobial resistance arising from the use of antimicrobials in animals
Aquatic Animal Health Code
Chapter 6.1 Introduction to the recommendations for controlling antimicrobial resistance
Chapter 6.2 Principles for responsible and prudent use of antimicrobial agents in aquatic animals
Chapter 6.3 Monitoring of the quantities and usage patterns of antimicrobial agents used in aquatic animals
Chapter 6.4 Development and harmonisation of national antimicrobial resistance surveillance and monitoring programmes for aquatic animals
Chapter 6.5 Risk analysis for antimicrobial resistance arising from the use of antimicrobial agents in aquatic animals
Terrestrial Animal Health Manual
Chapter 2.1.1 – Laboratory methodologies for bacterial antimicrobial susceptibility testing
Strengthening knowledge on AMR through monitoring and research
We base our work on the latest scientific evidence. Our action to curb antimicrobial resistance is no exception. In addition to animal health data, we have also been collecting information on the use of antimicrobials (AMU) in animals since 2015. Building the ANImal antiMicrobial USE (ANIMUSE) Global Database is a key component of our strategy to curb AMR. By facilitating national, regional, and global monitoring of antimicrobial use, this centralised database system helps countries monitor the effectiveness of interventions to reduce and optimise the use of antimicrobials over time.
A report has been published every year since 2016 to provide access to this crucial and growing set of information and has highlighted steady improvements in the animal health sector worldwide. Global quantities of antimicrobials used in animals, adjusted by animal biomass (and measured in mg/kg), have decreased by 13% worldwide between 2017 and 2019 (trends obtained from data reported by 80 participating countries). Adjusting antimicrobial quantities by animal biomass is key to draw relevant comparisons of the amounts of drugs used over time, across regions and sectors.
Responsible use of antibiotics is crucial to avoid the development and spread of drug-resistant bacteria, as is disease prevention, through actions such as vaccination and biosecurity measures. However, all possible solutions must be explored. For this reason, we are also a reliable partner of worldwide efforts to develop alternatives to antibiotics. We coordinate animal health research at a global level, and act as a prominent advocate for the development of alternatives to antimicrobials for treating sick animals.
WOAH considered the experience and feedback from Members and annually updates the template and guidance document based on requests for clarification from responding Members. The current version of these documents, are available below.
-
.pdf – 7 MB See the document
-
.pdf – 545 KB See the document
Disseminating knowledge about AMR and its solutions
Climate change, pandemic preparedness, antimicrobial resistance… for all global challenges, public awareness is a necessary first step that leads to change. And the growing threat of dangerous infections caused by drug-resistant pathogens for which there are few or no therapeutic options available may be one of the most overlooked global health threats of our time.
Drug-resistant infections can affect everyone. This is why we actively contribute to the global public conversation on antimicrobial resistance through social media, events jointly organised with our Quadripartite partners like the World AMR Awareness Week (WAAW), as well as through our participation in political fora.
Awareness leads to action, and action is a necessary path towards change.
United in the fight against antimicrobial resistance
No single organisation can tackle the global problem of AMR alone. This multifaceted challenge can only be met through a One Health approach, which considers animal, human, plant and environmental health as interconnected and interdependent. That is why the World Organisation for Animal Health (WOAH) has partnered with the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO), and more recently, with the United Nations Environment Programme (UNEP). Together, we have created the Quadripartite, a unique partnership to fight AMR and other health threats at the animal-human-environment interface.
As the global authority on animal health, we have embraced our role as a key coordinator of actions in the multisectoral global response to AMR with our Quadripartite partners. Providing a strategic approach as well as guidelines and recommendations to all stakeholders that produce, distribute, and administer antimicrobials enables us, alongside partner organisations, to foster efforts at country level and maximise impact and results.
A One Health response to AMR
The growing threat of antimicrobial resistance is one of the best examples of how dangerous pathogens, such as drug-resistant bacteria, viruses, fungi, and parasites, can spread between animals, humans, plants and within the environment.
Through the Quadripartite collaboration (FAO, UNEP, WHO, WOAH), we create synergy in our different areas of expertise and strive to mobilise public and private stakeholders, governments, and public opinion. Our actions include developing the capacity of Members for surveillance of antimicrobial use and AMR, ensuring consistency across the standard-setting activities of our organisations, evaluating and managing risks linked to AMR worldwide, and raising awareness through a united voice, notably during the World Antimicrobial Awareness Week (WAAW).
The One Health approach has become increasingly relevant in today’s world. Climate change, globalisation, and evolving human habits make it easier for pathogens to spread rapidly across different species and all regions of the world. Ensuring a responsible use of antimicrobials in all sectors makes the world safer for all.
Building a global governance on AMR
Because AMR needs to be tackled on so many different fronts, decisions must be made at a global level.
In 2019, the call for coordinated worldwide action reached the summit of global governance as the report “No Time to Wait: Securing the Future from Drug-Resistant Infections” was delivered to the Secretary-General of the United Nations. The report was drafted by the Interagency Coordination Group on Antimicrobial Resistance (IACG). This ad hoc group was mandated to provide practical guidance for ensuring sustained effective action to address AMR. The document was devised in consultation with the Tripartite (FAO, WHO, WOAH) and built upon the WHO’s 2015 Global Action Plan on Antimicrobial Resistance (GAP), in which WOAH was involved. Among others, the report called for the creation of:
The Global Leaders Group on antimicrobial resistance (GLG)
consisting of world leaders and experts from across sectors working together to accelerate political action on AMR. The group performs an independent global advisory and advocacy role. It works to maintain urgency, public support, political momentum and visibility of the AMR challenge on the global health and development agenda. It does this by collaborating with governments, international organisations, civil society, and the private sector. It advocates for prioritised political actions to mitigate drug resistant infections through responsible use of antimicrobials.
The Multi-Partner Trust fund (AMR MPTF)
coordinated by the Quadripartite collaboration. The AMR MPTF is the main mechanism to secure consistent and coordinated financing to support One Health national action plans and Tripartite workplans in a number of countries.
The Multi-stakeholder Partnership Platform
a constituency-based partnership platform facilitated and managed by the Quadripartite agencies with diverse representation (e.g. governments, private sector and civil society representing human, animal, plant and environment health, as well as agriculture and food and feed production). Its mission is to drive the development of a shared global vision on AMR, push for action to curb its spread, and enable the production of knowledge on AMR.
Today, advocacy, cooperation, funding and implementation of action plans at global, regional and country levels all follow the guiding vision of the Strategic Framework for collaboration on antimicrobial resistance. This framework was published by the Quadripartite in 2022. Its goal mirrors the objectives of the Global Action Plan: preserving antimicrobial efficacy and ensuring sustainable and equitable access to these drugs. All them while fostering their responsible use. The framework clearly defines goals, desired impact at country level, intermediate outcomes, and the paths towards those objectives for each sector.
Key political advocacy actions
The commitment of politicians and decision-makers is needed to tackle antimicrobial resistance. This is why the World Organisation for Animal Health and its partners have continuously worked to steer political attention towards AMR, its risks and solutions. We foster political action through, among others, our participation in high-level fora:
Global Health Security Agenda (GHSA)
- Since 2014 we participate, as an advisor, to the world Steering Committee of the GHSA, a joint endeavour among more than 40 countries that aims to accelerate progress towards a healthier world.
- The GHSA supports AMR efforts in the political space by keeping this topic on the agenda at the highest political levels at multiple fora and across sectors.
- The GHSA provides guidance and shares best practices to assist members in developing their capacity to address AMR.
United Nations General Assembly (UNGA)
- In September 2016, after a meeting summoned by the President of the UN General Assembly, UN members adopted a political declaration calling for action to address AMR at national and international levels.
- The meeting’s goal was, among others, to maintain strong national, regional and international political commitment in addressing antimicrobial resistance.
- Since the meeting, curbing AMR has been recognised as one of the necessary objectives to reach the UN’s Sustainable Development Goals (SDGs). The topic will be addressed for the second time in the UNGA in September 2024.
G20
- Since 2017, AMR is among the top priority health topics at the G20.
- This strategic multilateral platform connects the world’s major developed and emerging economies.
- Currently, the World Organisation for Animal Health and other Quadripartite members are active participants in a specific AMR working group at the G20. Through this working group, we are defining actions that G20 Members can take to help curb AMR.
What can you do to fight antimicrobial resistance?
The world needs antimicrobials for health, livelihood and food security, but they are losing their efficacy because of the evolution of antimicrobial resistance. As a main entry point of antimicrobials into the system, you are key participants in the solution to this global threat.
Find out what you can do to help mitigate antimicrobial resistance
You are:
A veterinary authority
An animal health or aquatic animal health professional
A farmer or aquatic animal producer
Part of the feed industry
Part of the pharmaceutical industry
You are a veterinary authority
Here is what you can do:
Advocate for the development of Public-Private Partnerships to support the implementation of good practices for the manufacturing, distribution, sales and use of antimicrobials in animals.
Make sure that guidelines for responsible use of antimicrobials are available in your country. Use WOAH’s international Standards and list of antimicrobials of veterinary importance to inform the development of these guidelines.
Put together One Health initiatives by collaborating with human health, environment and agriculture departments to fight AMR.
See more guidelines
-
.pdf – 84 KB See the document
-
.pdf – 132 KB See the document
Your main resources
-
Global Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials in Animals
-
List of antimicrobials of veterinary importance
-
Science-based international Standards
-
How to talk about antimicrobial resistance
You are an animal health or aquatic animal health professional
As an animal health or aquatic animal health professional, you are at the forefront of the fight against antimicrobial resistance. Misuse and overuse of antimicrobials in terrestrial and aquatic animals can lead to the development of resistant pathogens and undermine global health. As you have the power to prescribe and use antimicrobials, you have an essential role to play. Let’s preserve the efficacy of antimicrobials by using them responsibly and only when necessary.
Here is what you can do:
Educate terrestrial and aquatic animal producers on good biosecurity and husbandry practices to reduce the disease burden in animal populations and, therefore, the need for antimicrobials.
Evaluate all the therapeutic or hygienic alternatives to antimicrobials. Prescribe antimicrobials only when necessary, when no other treatment is possible or when it is the best possible option.
Check local guidelines to select relevant drugs for use in terrestrial or aquatic animals before prescribing antimicrobials.
See more guidelines
-
.pdf – 165 KB See the document
-
.pdf – 108 KB See the document
-
.pdf – 3 MB See the document
-
.pdf – 171 KB See the document
Your main resources
-
Global Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials in Animals
-
Aquatic Animal Health Strategy
-
List of antimicrobials of veterinary importance
-
Science-based international Standards
You are a farmer or aquatic animal producer
As a farmer or an aquatic animal producer, you have a key role in preserving the efficacy of antimicrobials worldwide. Whether it be by implementing appropriate disease prevention measures to reduce the need to administer antimicrobials to your animals, or by making sure that, when you do use antimicrobials, you do it responsibly in consultation with your veterinarian/aquatic animal health professional. Furthermore, your actions have an impact on global health, as drug resistant pathogens could spread between animal, human and plant populations.
Here is what you can do:
Follow good farming and/or husbandry practices to avoid stress in your terrestrial and aquatic animals, which lower their natural defences.
Adopt hygiene procedures and biosecurity principles to prevent the introduction and spread of pathogens which could place your animals’ health and your livelihood at risk.
Vaccinate your animals to reduce the need for antimicrobials and the related costs. Vaccines, when available, can provide life-long immunity to your animals for certain diseases.
See more guidelines
-
.pdf – 170 KB See the document
-
Brochure
Fighting AMR as a farmer
.pdf – 110 KB See the document -
.pdf – 165 KB See the document
-
.pdf – 111 KB See the document
Your main resources
-
Global Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials in Animals
-
Aquatic Animal Health Strategy
-
List of antimicrobials of veterinary importance
-
Science-based international Standards
You are a feed manufacturer or supplier
As a feed manufacturer or supplier, you have a key role to play in curbing the rise of antimicrobial resistance by ensuring that the products you market are safe, compliant with regulations, and that they reach responsible and informed users.
Here is what you can do:
Follow all legal requirements as well as best practices and international Standards for the manufacturing and distribution of medicated feeds.
Prevent cross-contamination of non-medicated feed with antimicrobials.
Label your products with the appropriate information to ensure effective and safe use. This includes level of medication, approved claim, intended species, directions for use, withdrawal periods, warning and cautions.
See more guidelines
-
.pdf – 78 KB See the document
-
.pdf – 133 KB See the document
Your main resources
-
Global Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials in Animals
-
Aquatic Animal Health Strategy
-
Science-based international Standards
You are an antimicrobial manufacturer
As a stakeholder of the pharmaceutical industry, you have a key role in steering antimicrobial manufacturing, distribution, sales and use worldwide, whether it be by helping develop alternatives to antimicrobials, by supporting the circulation of quality medicines or by providing authorities with data on their use.
Here is what you can do:
Support and conduct research for the development of cost-effective vaccines and other alternative medicines that can reduce the need for antimicrobials and create new business opportunities for your company.
Give worldwide access to rapid, affordable, accurate and reliable diagnostic tests to improve disease detection, and enable the prescription and use of appropriate treatments.
Ensure that pharmaceutical waste derived from your activities is properly disposed of to avoid spillover of antimicrobial residues and other contaminants into the environment.
See more guidelines
-
.pdf – 75 KB See the document
-
.pdf – 62 KB See the document
Your main resources
-
Global Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials in Animals
-
Aquatic Animal Health Strategy
-
Science-based international Standards
Are you are a concerned citizen?
Do you produce, use or prescribe antimicrobials?
Do you want to learn more about our multisectoral initiatives?
Events
-
Global Events, High-level advocacy
United Nations General Assembly: High-Level Meeting on AMR
Highlight -
Global Events
Securing our future from AMR – Global Youth Dialogue
-
Global Events
World AMR Awareness Week 2023
You are a concerned citizen: learn more about AMR and spread the word
-
.pdf – 136 KB See the document
-
.pdf – 620 KB See the document
You produce, use or prescribe antimicrobials: improve your practice and spread the word
-
Infographic
ANIMUSE: Benefits for Everyone
.pdf – 54 KB See the document -
Guidelines
How to talk about antimicrobial resistance
.pdf – 416 KB See the document -
.pdf – 165 KB See the document
-
.pdf – 108 KB See the document
-
.pdf – 171 KB See the document
-
.pdf – 165 KB See the document
-
.pdf – 111 KB See the document
-
.pdf – 170 KB See the document
-
Brochure
Fighting AMR as a farmer
.pdf – 110 KB See the document -
.pdf – 3 MB See the document
-
.pdf – 78 KB See the document
-
.pdf – 133 KB See the document
-
.pdf – 75 KB See the document
-
.pdf – 62 KB See the document
-
.pdf – 84 KB See the document
-
.pdf – 132 KB See the document
You want to learn more about AMR and how to curb it
-
Policy paper, Position statement
Use of antimicrobials as growth promoters: WOAH urges Veterinary Authorities and the animal industry to live up to their commitments
.pdf – 58 KB See the document -
.pdf – 7 MB See the document
-
.pdf – 309 KB See the document
-
.pdf – 35 KB See the document
-
Report, Working Group
List of Antimicrobial Agents of Veterinary Importance
.pdf – 309 KB See the document -
Report, Working Group
WOAH Standards, Guidelines and Resolutions on AMR and the use of antimicrobial agents
.pdf – 453 KB See the document
-
Former Annual Reports on Antimicrobials Agents Intended for Use in Animals
-
WOAH Bulletin
-
Scientific and Technical reviews
-
ANIMUSE – Global database on ANImal antiMicrobial USE
You want to learn more about our multisectoral initiatives
-
.pdf – 8 MB See the document
-
.pdf – 1 MB See the document
-
.pdf – 606 KB See the document
-
Infographic
One Health Priority Research Agenda for AMR
.pdf – 783 KB See the document -
Factsheet
AMR MPFT Frequently asked questions
.pdf – 151 KB See the document -
.pdf – 874 KB See the document
-
.pdf – 17 MB See the document
-
.pdf – 1 MB See the document
-
.pdf – 639 KB See the document
-
Strategic Plan
Strategic framework for collaboration on antimicrobial resistance – together for One Health
.pdf – 355 KB See the document
You are a journalist
News
-
-
-
Published on 15/11/2023
-
Published on 07/09/2023
-
Published on 01/06/2023
-
News
Global antimicrobial resistance forum launched to help tackle common threat to planetary health
Published on 06/04/2023 -
Published on 21/02/2023
-
Joint Press Release
Quadripartite welcomes new political commitments in fight against antimicrobial resistance
Published on 25/11/2022 -
Joint Press Release
One Health Joint Plan of Action launched to address health threats to humans, animals, plants and environment
Published on 17/10/2022 -
Joint Press Release
The Global Leaders Group host side event at UN General Assembly on Antimicrobial Resistance (AMR)
Published on 22/09/2022
World Antibiotic Awareness Week (WAAW)
-
Global Events
World AMR Awareness Week 2023
-
Global Events
World Antimicrobial Awareness Week 2022
-
Global Events
World Antimicrobial Awareness Week 2021
-
Global Events
Securing our future from AMR – Global Youth Dialogue
