Aquatic Animal Health Code
Aquatic Animal Health Code |
Introduction
The importation of aquatic animals and animal products, whether of aquatic or terrestrial origin, involves a degree of disease risk to the importing country. This risk, which may be to humans or animals, may be represented by one or several diseases not present in the importing country.
The principal aim of import risk analysis is to provide importing countries with an objective and defensible method of assessing the disease risks associated with the importation of animals, animal products, animal genetic material, feedstuffs, biological products and pathological material. The principles and methods are the same whether the commodities are derived from aquatic and/or terrestrial animal sources. The analysis should be transparent. This is necessary so that the exporting country is provided with clear reasons for the imposition of import conditions or refusal to import.
Transparency is also essential because data are often uncertain or incomplete and, without full documentation, the distinction between facts and the analyst's value judgements may blur.
This chapter outlines the role of the OIE with respect to the Agreement on the Application of Sanitary and Phytosanitary Measures (the so-called SPS Agreement) of the World Trade Organization (WTO) and describes the OIE procedure for settlement of disputes.
Chapter 2.2. provides recommendations and principles for conducting transparent, objective and defensible risk analyses for international trade. However, it cannot provide details on the means by which a risk analysis is carried out as the purpose of the Aquatic Code is simply to outline the necessary basic steps. The components of risk analysis described in Chapter 2.2. are hazard identification, risk assessment, risk management and risk communication (Figure 1).
Fig. 1. The four components of risk analysis
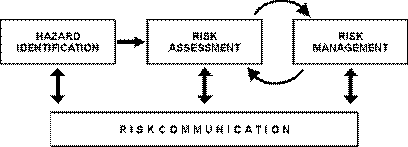
The risk assessment is the component of the analysis that estimates the likelihood and consequences associated with a hazard. Risk assessments may be qualitative or quantitative. For many diseases, particularly those referred to in the Aquatic Code where there are well developed internationally agreed standards, there is broad agreement concerning the likely risks, although the status of some diseases may differ between countries or even between the Northern and Southern Hemispheres. In many cases it is likely that a qualitative assessment is all that is required. Qualitative assessment does not require mathematical modelling skills to carry out and so is often the type of assessment used for routine decision making. No single method of import risk assessment has proven applicable in all situations, and different methods may be appropriate in different circumstances.
The process of import risk analysis on aquatic animals and aquatic animal products usually needs to take into consideration the results of an evaluation of the Competent Authorities, zoning and regionalisation, and surveillance systems that are in place for monitoring aquatic animal health in the exporting country. These are described in separate chapters in the Aquatic Code.
The Agreement on the Application of Sanitary and Phytosanitary Measures and role and responsibility of the OIE
The SPS Agreement encourages WTO Members to base their sanitary measures on international standards, guidelines and recommendations, where they exist. Members may choose to adopt a higher level of protection than that provided by international texts if there is a scientific justification or if the level of protection provided by the relevant international texts is considered to be inappropriate. In such circumstances, Members are subject to obligations relating to risk assessment and to a consistent approach to risk management.
The SPS Agreement encourages Governments to make a wider use of risk analysis: WTO Members shall undertake an assessment as appropriate to the circumstances of the actual risk involved.
The SPS Agreement recognises the OIE as the relevant international organisation responsible for the development and promotion of international animal health standards, guidelines, and recommendations affecting trade in live animals and animal products, whether aquatic or terrestrial in origin.
The OIE in-house procedure for settlement of disputes
The OIE shall maintain its existing voluntary in-house mechanisms for assisting Members to resolve differences. In-house procedures that will apply are that:
Both parties agree to give the OIE a mandate to assist them in resolving their differences.
If considered appropriate, the Director General of the OIE recommends an expert, or experts, and a chairman, as requested, agreed by both parties.
Both parties agree on the terms of reference and working programme, and to meet all expenses incurred by the OIE.
The expert or experts are entitled to seek clarification of any of the information and data provided by either country in the assessment or consultation processes, or to request additional information or data from either country.
The expert or experts should submit a confidential report to the Director General, who will transmit it to both parties.
2009 ©OIE Aquatic Animal Health Code |